From what alkene could each of the following glycols be prepared by the OsO 4 or KMnO
Question:
From what alkene could each of the following glycols be prepared by the OsO4 or KMnO4 method?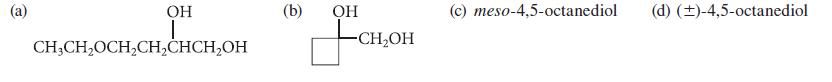
Transcribed Image Text:
OH I CH3CH₂OCH₂CH₂CHCH₂OH (b) OH -CH₂OH (c) meso-4,5-octanediol (d) (±)-4,5-octanediol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a b The alkene required is 4ethoxy1butene CH3CHOCH...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From which alkene could each of the following cyclopropane derivatives be prepared using the Simmons- Smith reaction? CH3
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
What alkene should be used to synthesize each of the following alkyl bromides? a. b. c. d. CHa CH3CCH Br CH2CHCH3 Br CH3 CCH 2 CH2CH3
-
1. Calculate the budgeted nights booked: Maximum capacity (30 rooms) * Number of days per year (365) * Expected occupancy rate (80%) = 8760 nights. 2. Calculate the tariff revenues: Budgeted nights...
-
Do all transactions by U.S. companies with foreign parties require special accounting procedures by the U.S. companies? Explain.
-
Explain why Aggregate Planning and Master Scheduling factor into the overall Inventory Management process? How does it impact the business?
-
2-4. What is the difference between an organizations business and its goals?
-
CVP analysis, margin of safety Suppose Latin Corp.s breakeven point is revenues of $1,500,000. Fixed costs are $600,000. 1. Compute the contribution margin percentage. 2. Compute the selling price if...
-
Note: Next to each item, select yes or no to indicate whether th Current Asset Item 1. Cash and cash equivalents Yes 2. Property plant & equipment No 3. Inventory Yes 4. Accounts payable No 5....
-
Show a curved-arrow mechanism for the first step, and the structure of the cyclic intermediate formed, when an alkene is treated with KMnO 4 . A Lewis structure for the permanganate ion is as...
-
Predict the major product(s) of each of the following transformations. (a) Et C-CH + CHOH (solvent) ***** H Et (optically active) HSO4 (trace) HSO4 (b) The enantiomer of the epoxide in part (a) +...
-
List three separate motivations for shopping, giving an example of each.
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
Charlene wrote a letter to Rachel offering to sell her car, a Proton Saga, for RM 60,000. The letter reached Rachel on 25. 11.2020. Rachel sent her letter of acceptance at 3 p.m. on the same day....
-
Data for the risk premium sensitivities (b, s, and h) as well as the beta coefficient for the CAPM of two companies are listed in the following table: Company b s h ERP SMBP HMLP Beta Alpha 1.1114...
-
Free-Response Questions 1. m Initial position eviribrA ARAL m Incline raised to 0 <0max pr A block of mass m is initially at rest on a rough board, which is initially horizontal on a tabletop. The...
-
A picture frame sits atop a bookshelf. When the bookshelf is bumped, the frame tumbles to the floor, landing after 0.64 s. How tall is the bookshelf?
-
From a product identity, we obtain Cos x/2 cos x/4 = 1/2 [cos(3/4 x) + cos(1/4 x)] Find the corresponding sum of cosines for cos x/2 cos x/4 cos x/8 cos x/16 Do you see a generalization?
-
An auto-parts manufacturer is considering establishing an engineering computing center. This center will be equipped with three engineering workstations each of which would cost $25,000 and have a...
-
Cardiolipins arc a group of lipids found in heart muscles. What products would be formed if all ester bonds, including phosphates, were saponified by treatment with aqueousNaOH? ROCH2 CH2OOR" A...
-
Stearolic acid, C18H32O2 yields stearic acid on catalytic hydrogenation and undergoes oxidative cleavage with ozone to yield nonanoic acid and nonanedioic acid. What is the structure of Stearolic...
-
How would you synthesize Stearolic acid (Problem 27.19) from 1-dccyne and 1-chloro-7-iodohcptane?
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


