(S)-(1)-Aspartic acid is one of the naturally occurring a-amino acids. Over time in the environment or in...
Question:
(S)-(1)-Aspartic acid is one of the naturally occurring a-amino acids.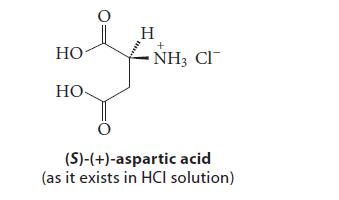
Over time in the environment or in aqueous solution, aspartic acid can undergo racemization very slowly (by a process that we won’t consider here). The racemization of aspartic acid occurs at a known rate that can be used to date tissue samples in forensic investigations. The rate of racemization in skull samples follows the equation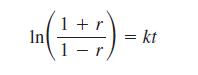
where k 5 6.24 3 10–4 yr–1 and t 5 age of the sample in years. In this equation, r is the ratio of (R)-(2)- and (S)-(1)-aspartic acid.
The local coroner’s office, knowing your expertise in organic chemistry, comes to you with an old skull sample that was excavated from a building site. You isolate partially racemized aspartic acid from the sample and find (by chiral chromatography) that the sample contains 6% of the (R)-(2)-enantiomer and 94% of the (S)-(1)-enantiomer.
(a) How old is the skull sample?
(b) What is the enantiomeric excess of the (S)-(1)-enantiomer in the sample?
(c) If (S)-(1)-aspartic acid has a specific rotation [a]20D 5 124.5 degrees mL g–1 dm–1 in 6 M aqueous HCl solution, what is the observed rotation of 100 mg of the forensic (partially racemized) sample in 10 mL of 6 M aqueous HCl?
Step by Step Answer:






