State whether each of the following molecules is achiral or chiral. Cl H-C-Br -EL F (b) CI
Question:
State whether each of the following molecules is achiral or chiral.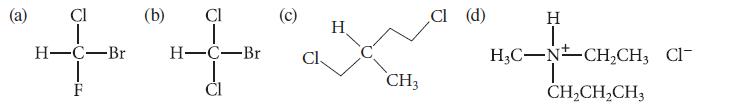
Transcribed Image Text:
Cl H-C-Br -EL F (b) CI H-C-Br Cl H CH3 Cl (d) H I H₂C-NCH₂CH, Cl- T CH₂CH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a This compound is chiral b This compound is achiral c Thi...View the full answer

Answered By

WAHIDUL HAQUE
hello,
I'm a professional academic solution provider working as a freelance academic solution provider since 7 years. I have completed numerous projects. Help lots of students to get good marks in their exams and quizzes. I can provide any type of academic help to your homework, classwork etc, if you are a student of Accounting, Finance, Economics, Statistics. I believe in satisfying client by my work quality, rather than making one-time profit. I charge reasonable so that we make good long term relationship. why will you choose me? i am an extremely passionate, boldly honest, ethically driven and pro-active contractor that holds each of my clients in high regards throughout all my business relations. in addition, I'll always make sure that I'm giving my 100% better in every work that will be entrusted to me to be able to produce an outcome that will meet my client's standards. so if you are a student that is now reading my profile and considering me for your academic help. please feel free to look through my working history, feedback and contact me if you see or read something that interests you. I appreciate your time and consideration.
regards
4.90+
233+ Reviews
368+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Indicate whether each of the following statements is true or false. If false, explain why. (a) In some cases, constitutional isomers are chiral. (b) In every case, a pair of enantiomers have a mirror...
-
Indicate whether each of the following statements is true or false. If false, explain why. (a) In every case, pair of enantiomers have a mirror- image relationship. (b) If a compound has an...
-
Identify whether each of the following compounds is chiral or achiral: a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. CI
-
The 10-year Coupon Bond has a face value of $1,000, the annual coupon rate is 5 percent (out of its face value), the yield to maturity is 10 percent. (2.a) show me the cash flows of this coupon bond,...
-
Could a plaintiff win such a case without showing that she had contracted the HIV virus?
-
Very small crystals composed of 1000 to 100,000 atoms, called quantum dots, are being investigated for use in electronic devices. (a) A quantum dot was made of solid silicon in the shape of a sphere,...
-
What is the probability of randomly generating your cousins telephone number?
-
A binary mixture of mole fraction zj is flashed (o conditions T and P, Fur one of the following determine: the equilibrium mole fractions x1 and y1 of the liquid and vapor phases formed, the molar...
-
Dewey Cheatham & Howe is a law firm. Among its February transactions were the following: Date Transaction 2/7 Provided legal services to Jones Company on account. Dewey's lawyers spent 100 hours on...
-
Determine the stereochemical configuration of the following enantiomer of 3-chloro-1-pentene: HC CH Cl I CH CHCH3
-
Identify the asymmetric carbon(s) in 4-methyloctane: CH3 CH3CHCHCHCHCHCHCH3 4-methyloctane
-
A farmer runs a heat pump with a 2 kW motor. It should keep a chicken hatchery at 30oC, which loses energy at a rate of 10 kW to the colder ambient Tamb. What is the minimum coefficient of...
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
Distinguish among first-, second-, and third-degree bums.
-
The following selected information was taken from Sun Valley Citys general fund statement of revenues, expenditures, and changes in fund balance for the year ended December 31, 2019: Revenues:...
-
Treatment of 1, 1-diphenyl- 1, 2-epoxyethane with aqueous acid yields diphenylacetaldehyde as the major product. Propose a mechanism for the reaction. Ph H30+ PHCHCH Ph Ph
-
How would you prepare o-hydroxyphenyl-acetaldehyde from phenol? More than one step is required. HO o-Hydroxyphenylacetaldehyde CH-CO
-
Imagine that you have treated (2R, 3R)-2, 3-epoxy-3-methylpentane with aqueous acid to carry out a ring-opening reaction. (a) Draw the epoxide, showing stereochemistry. (b) Draw and name the product,...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


