Suggest a structure for a constitutional isomer of the following compound that should have greater water solubility,
Question:
Suggest a structure for a constitutional isomer of the following compound that should have greater water solubility, and explain your reasoning. The structure should not be an enol, because enols are not stable. 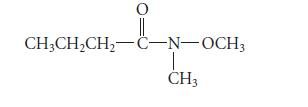
Transcribed Image Text:
O || CH3CH₂CH₂-C-N-OCH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The compound shown in the image is Nmethylpropanamide A constitutional isomer of this compound ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer...
-
our class. The word count of the text that includes your "own words" should be at least 500 words (about 700 max.). The text should be typed double-spaced using a 12-point font size in Times New...
-
(a) Two amides are constitutional isomers and have the formula C 4 H 9 NO, and each contains an isopropyl group as part of its structure. Give structures for these two isomeric amides. (b) Draw the...
-
Cost-Volume-Profit (CVP) analysis can be used to determine the effect of changes in costs and volumes on a company's net profits. This project assignment assumes that you are newly hired as an...
-
Identify the questions you should ask to anticipate your audiences reaction.
-
The cantilever beam AB shown in the figure is subjected to a triangular load acting throughout one-half of its length and a concentrated load acting at the free end. Draw the shear-force and...
-
What is the retention rate of people in the position for which I am interviewing?
-
Boehm Incorporated is expected to pay a $1.50 per share dividend at the end of this year (i.e., D1 = $1.50). The dividend is expected to grow at a constant rate of 7% a year. The required rate of...
-
You are an analyst in the Utilities sector who uses sector multiples to determine price targets for individual utility companies. Utilities have an average multiple of 19. TRY Energy has 10,000,000...
-
Vitamins can be classified as fat-soluble or watersoluble. Fat-soluble vitamins can be stored in fatty tissues, whereas water-soluble vitamins can be excreted in the urine. (a) The structures of some...
-
When salad oil is mixed with water and shaken, two layers quickly separate, the oily layer on top and the water layer on the bottom. When an egg yolk (which is rich in lecithin, a phospholipid) is...
-
Figure shows a circuit diagram. Determine (a) The current, (b) The potential of wire A relative to ground, and (c) The voltage drop across the 1 500-? resistor. I 000 N 1 500 N 25.0 V 30.0 V 2 000 N...
-
Consider how they might directly apply to your life and work environment when answering the questions below. Competency 1: Evaluate data-driven processes and approaches of an organization's...
-
There are several website optimizer tools available to help you "increase website conversion rates." Following : Explain fully what is meant by "increase website conversion rates"; then, identify two...
-
Imagine being a human resource director for a large hotel chain. Report to management highlighting problematic diversity issues that may arise. Identify 3 challenging diversity issues (e.g., race,...
-
A study based on a sample of 4 0 0 medical school students finds that the ratio of female students is 0 . 4 8 . The school rules require that the female ratio in the school is at least 0 . 5 ? a ) (...
-
One of the many paradoxes in leadership is the challenge of encouraging a team effort while simultaneously encouraging individuals to excel. Why is this paradox a challenge for team leaders, and how...
-
If a physician plans to obtain a sample of spinal fluid from a patient, what anatomical site can be safely used, and how should the patient be positioned to facilitate this procedure?
-
B made an issue of 150,000 $1 ordinary shares at a premium of 20% the proceeds of which is received by cheque. What is the correct journal to record this? A. Bank Share capital Share premium B. Bank...
-
Provide names for thesecompounds: a) CH;CH,CH,CH,CH,CH b) CH f) CH,CCH,CCH, d) CI g) h)
-
Draw the structures for these compounds: (a) (Z)-Oct-3-en-2-one (b) 3-Ethylheptanal (c) 2, 4-Pentadienal (d) 3, 4-Dimethylbenzaldehyde (e) 1-Phenyl-1-propanone (f) 2, 2, 6, 6-Ttramythleyelohexanone
-
Explain which the most acidic hydrogen's in these compoundsare c) CH,CCH,CH, b) PHCH CCH3
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


