The principles for predicting bond angles do not permit a distinction between the following two conceivable forms
Question:
The principles for predicting bond angles do not permit a distinction between the following two conceivable forms of ethylene.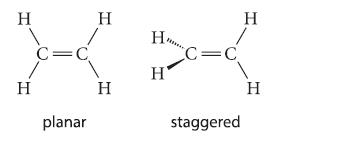
The dipole moment of ethylene is zero. Does this experimental fact provide a clue to the preferred dihedral angles in ethylene? Why or why not?
Transcribed Image Text:
H H C=C planar H H H₂ Н' C=C staggered H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
No matter how any CH group is turned the resultant bo...View the full answer

Answered By

Gilbert Chesire
I am a diligent writer who understands the writing conventions used in the industry and with the expertise to produce high quality papers at all times. I love to write plagiarism free work with which the grammar flows perfectly. I write both academics and articles with a lot of enthusiasm. I am always determined to put the interests of my customers before mine so as to build a cohesive environment where we can benefit from each other. I value all my clients and I pay them back by delivering the quality of work they yearn to get.
4.80+
14+ Reviews
49+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The principles for predicting bond angles do not permit a distinction between the following two conceivable forms of ethvlene. The dipole moment of ethylene is zero. Does this experimental fact...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Fresh" is a fresh fruit shopping chain. Their specialty is organically grown and seasonal fruits. They now operate about 10 outlets in Pune. Fruits are obtained from farmers within and near-by states...
-
Jin, the recruitment manager at Randents Inc., reviews the performance of his team members on a monthly basis. Based on the results of his monthly reviews, he decides to conduct daily reviews to...
-
How do business writers organize most informational reports, and what can writers assume about the audience?
-
A circular bar of length L = 32 in. and diameter d = 0.75 in. is subjected to tension by forces P (see figure). The wire is made of a copper alloy having the following hyperbolic stress-strain...
-
4 Cross-cultural adjustment is a multi-dimensional process. What are the different dimensions involved? What is the relationship between the different dimensions?
-
The accounts receivable for Pastors Brewing Company on March 31, 2016 was $18,000. Firm sales were roughly evenly split between credit and cash sales, with 40 percent of the credit sales collected in...
-
You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a comprehensive analysis of the company's financial statements,...
-
Three possible dihedral angles for H 2 O 2 (0, 90, and 180) are shown in Fig. 1.6. (a) Assume that the H 2 O 2 molecule exists predominantly in one of these arrangements. Which of the dihedral angles...
-
Account for the fact that H 3 CCl (dipole moment 1.94 D) and H 3 CF (dipole moment 1.82 D) have almost identical dipole moments, even though fluorine is considerably more electronegative than...
-
Oil prices have increased a great deal in the last decade. The table below shows the average oil price for each year since 1949. Many companies use oil products as a resource in their own business...
-
the assessment include developing gantt chart, work breakdown structure and and all task 3 are related to its respective task 2. all the instructions are given in the assignment itself. Assessment...
-
Mens heights are normally distributed with mean 68.6in. and standard deviation 2.8in. Air Force Pilots The U.S. Air Force required that pilots have heights between 64 in. and 77 in. Find the...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
ACC1810 - PRINCIPLES OF FINANCIAL ACCOUNTING Project 11: Chapter 11 - Stockholders' Equity Part B: Financial Statements The accounts of Rehearsal Corporation are listed along with their adjusted...
-
Match the term to the description. Outcome evaluation Focuses on the accomplishments and impact of a service, program, or policy and its effectiveness in attaining its outcomes set prior to...
-
Describe the organs of static and dynamic equilibrium and their functions.
-
Aztec Furnishings makes hand-crafted furniture for sale in its retail stores. The furniture maker has recently installed a new assembly process, including a new sander and polisher. With this new...
-
Show the products of thesereactions: . Cl Br . b) , a) CH;CH,CH,CH, + OH CI c) CH2=CHCH, +
-
Explain why only one of the two chlorines of 1, 2-dichloro-2-methylpropane is replaced by a hydroxy group when the compound is heated in water (see the preceding hydrolysis reaction.
-
On the basis of the bond cleavage shown for this reaction in Figure 10.1, predict the stereo chemistry of the product.Explain. OCCH, CH,CH, ." -
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


