The shape of one of the five energetically equivalent 3d orbitals follows. From your answer to Problem
Question:
The shape of one of the five energetically equivalent 3d orbitals follows. From your answer to Problem 1.33, sketch the nodes of this 3d orbital, and associate a wave peak or a wave trough with each lobe of the orbital.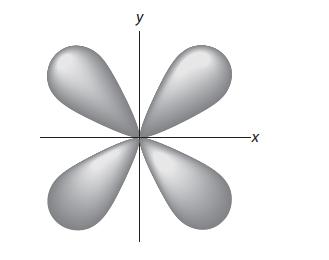
Problem 1.33
Two types of nodes occur in atomic orbitals: spherical surfaces and planes. Examine the nodes in 2s, 2p, and 3p orbitals, and show that they agree with the following statements:
An orbital of principal quantum number n has n - 1 nodes.
The value of ml gives the number of planar nodes.
How many spherical nodes does a 5s orbital have? A 3d orbital? How many nodes of all types does a 3d orbital have?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





