Use the curved-arrow notation to derive a structure for the product of each of the following Lewis
Question:
Use the curved-arrow notation to derive a structure for the product of each of the following Lewis acid–base association reactions; be sure to assign formal charges. Label the Lewis acid and the Lewis base, and identify the atom that donates electrons in each case.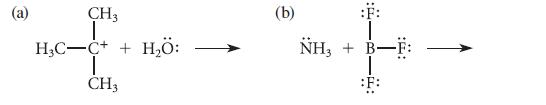
Transcribed Image Text:
(a) H₂C CH3 T C++ | CH3 H₂Ö: (b) NH3 + :F: -F:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a The product results from donation of an unshared electron pair from the oxy...View the full answer

Answered By

Leah Muchiri
I am graduate in Bachelor of Actuarial Science and a certified accountant. I am also a prolific writer with six years experience in academic writing. My working principle are being timely and delivering 100% plagiarized free work. I usually present a precised solution to every work am assigned to do. Most of my student earn A++ GRADE using my precised and correct solutions.
4.90+
52+ Reviews
125+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the curved - arrow notation to derive three other resonance structures for anthracene. CC1..thmatummail three additional structures anthracene
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
Use curved arrow notation to show the bonding changes in the reaction of cis-4-tert-butylcyclohexyl bromide with potassium tert-butoxide. Be sure your drawing correctly represents the spatial...
-
Tony acquired 1,000 shares in X Co (a resident public company) for $10 each in August 2000. In January this year X Co returned $7 of capital to its shareholder in respect to each share they held. The...
-
1. Suppose a plaintiff sues a defendant, claiming that the defective design of a power saw caused him serious injury. At trial, the defendants expert witness is an engineer who is acknowledged as the...
-
Commercial concentrated aqueous ammonia is 28% NH3 by mass and has a density of 0.90 g/mL. What is the molarity of this solution?
-
1. You are valuing DistressCo, a company struggling to hold market share. The company currently generates $120 million in revenue but is expected to shrink to $100 million next year. Cost of sales...
-
On February 1, the Miro Company needs to purchase some office equipment. The company is presently short of cash and expects to be short for several months. The company treasurer has indicated that he...
-
Determining Merchandise to be Included or Excluded from Ending Inventory The unadjusted inventory balance of Sara Ann Corp. is $450,000 on December 31, 2020, based on a physical inventory count. The...
-
(a) Two amides are constitutional isomers and have the formula C 4 H 9 NO, and each contains an isopropyl group as part of its structure. Give structures for these two isomeric amides. (b) Draw the...
-
The a-amino acids are the building blocks of proteins. Most have the following general structure: These amino acids differ only in their side chains R. What functional groups are present in the side...
-
In Exercises 25-28, use the true statements below to determine whether you know the conclusion is true or false. Explain your reasoning. If Arlo goes to the baseball game, then he will buy a hot dog....
-
Use Table 19-4 to calculate the building, contents, and total property insurance premiums for the policy (in $). Area Structural Rating Class Building Value 4 B $86,000 $ Building Premium Contents...
-
What are some reasons why leadership theory has evolved? Which theory of leadership is most applicable to today's organizations? Identify a leader that you admire and answer the following: What makes...
-
Identifying one major OSHA standard and one EPA law that are important to aviation and discussing how each has improved aviation safety
-
What is network optimization and what are some of the best practices that are used in the industry to optimize networks? Also, why is network documentation important and what are the security...
-
Demonstrate your understanding of data types by examining a public dataset and identifying the NOIR analytical data types of each of the data field (variables). This skill will be used frequently in...
-
Write complete sentences using each of the following terms to correctly describe the relative locations of specific body parts: a. Superior b. Inferior c. Anterior d. Posterior e. Medial f. Lateral...
-
Charles owns an office building and land that are used in his trade or business. The office building and land were acquired in 1978 for $800,000 and $100,000, respectively. During the current year,...
-
The Meerwein?Ponndorf?Verley reaction involves reduction of a ketone by treatment with an excess of aluminum triisopropoxide. The mechanism the process is closely related to the Cannizzaro reaction...
-
Propose a mechanism to account for the formation of 3, 5-dimethylpyrazole from hydrazine and 2, 4-pcntancdionc. Look carefully to see what has happened to each carbonyl carbon in going from starting...
-
In light of ?our answer to Problem 19.56, propose a mechanism for the formation of 3, 5 -dimethylisoxazole from hydroxylamine and 2, 4-pentanedione. CH 3,5-Dimethylisoxazole
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


