When trans-3-hexene is subjected to ozonolysis in the presence of an excess of acetaldehyde containing the isotope
Question:
When trans-3-hexene is subjected to ozonolysis in the presence of an excess of acetaldehyde containing the isotope 18O, an ozonide is isolated that contains the isotope at one of the oxygens. Use the mechanism of ozonolysis to postulate a structure for the ozonide, including the position of the isotope.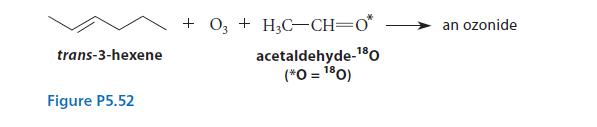
Transcribed Image Text:
trans-3-hexene Figure P5.52 +03+H3C-CH=0* acetaldehyde-¹80 (*0 = 180) an ozonide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
The addition of ozone to trans3hexene gives an initial cycloaddition product ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the presence of an acid catalyst, acetaldehyde forms a trimer known as paraldehyde. Because it induces sleep when it is administered to animals in large doses, paraldehyde is used as a sedative or...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
When 1, 4-dioxane is heated in the presence of HI, compound A is obtained: a. Draw the structure of compound A. b. If one mole of dioxane is used, how many moles of compound A are formed? c. Show a...
-
What sort of debates or experiences would get overlooked if professionals and researchers ignored these distinctions? How would you classify the following? An assigned expatriate who falls in love...
-
Blasingham Company is currently manufacturing Part Q108, producing 35,000 units annually. The part is used in the production of several products made by Blasingham. The cost per unit for Q108 is as...
-
Write SQL commands for the following: a. Create two different forms of the INSERT command to add a student with a student ID of 65798 and last name Lopez to the Student table. b. Now write a command...
-
What if I cant afford to pay you? If an organization wont help you because you cant afford to pay, go somewhere else for help.
-
The Green Company produces chemicals in a perfectly competitive market. The current market price is $40; the firms total cost is C = 100 + 4Q + Q2. a. Determine the firms profit-maximizing output....
-
Failure to record a sales order in the amount of $3,000 could be detected by reconciling input to which of the following batch totals? Choose all that apply. Select one or more: O a. Hash total on...
-
Isobutylene (2-methylpropene) can be polymerized by treating it with liquid HF as shown in Fig. P5.53. A small amount of tert-butyl fluoride is formed in the reaction. Suggest a curved-arrow...
-
Equations 5.25ac on p. 193 show the formation of trialkylboranes from alkenes and BH 3 . In the reaction of 2,3-dimethyl-2-butene with BH 3 , only two equivalents of the alkene react, even with a...
-
What factors increase the benefits of accelerating deductions or deferring income?
-
The following information was obtained from the records of Shae Inc.: Merchandise inventory $ 88,000 Notes payable (long-term) 100,000 Net sales 300,000 Buildings and equipment 168,000 Selling,...
-
Absent Clothing Company Savita Kapur, CEO, founded Absent Clothing Company (ACC) in 2005. ACC sells practical athletic wear to service the yoga and pilates market. Savita originally created ACC with...
-
Find the indicated area under the curve of the standard normal distribution; then convert it to a percentage and fill in the blank. About % of the area is between z = - 3.5 and z = 3.5 (or...
-
EM 605 Spring 2021 Midterm Exam 3/17/2021 The linear programming problem whose output follows is used to determine how many bottles of Hell-bound red nail polish (x1), Blood red nail polish (x2),...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Review Figure A-4 on page 19, and then state whether each of the following paired observations is on, above, or below the x axis and on, to the left of, or to the right of the y axis. a. (-10, 4) b....
-
The overall reaction and equilibrium constant value for a hydrogenoxygen fuel cell at 298 K is 2H 2 (g) + O 2 (g) 2H 2 O(l) K = 1.28 10 83 a. Calculate E cell and G 8 at 298 K for the fuel cell...
-
In these examples the additional structure or structures are not important contributors to the resonance hybrid for the compound represented by the first structure, explain. a) 8-8 c) CH-C=N: b) :0...
-
Draw the important resonance structures for these species and discuss the contribution of each to the resonance hybrid. Explain whether the species has a large or a small amount of resonance...
-
Explain why this carbocation is considerably more stable than this structure would suggest: H +C-0-CH, H
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


