Which compound in each of the sets shown in Fig. P22.64 is most acidic? Explain. (b) O
Question:
Which compound in each of the sets shown in Fig. P22.64 is most acidic? Explain.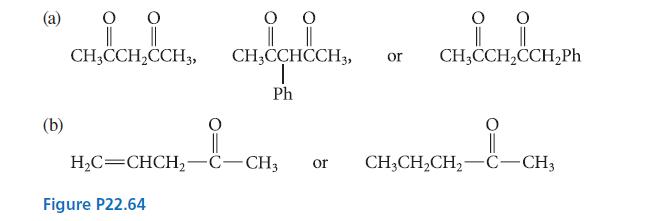
Transcribed Image Text:
(b) O O H₂C=CHCH₂- Figure P22.64 °° O O CH3CCHCCH3, or CH3CCH₂CCH₂Ph T Ph CH3CCH₂CCH3 CH₂C || -C-CH3 || or CH3CH₂CH₂-C-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The second compound is most acidic because its conjugatebase anion structure below has the greatest ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are Detroit's strengths and what are the pros and cons of living in detroit? Explain
-
Which compound in each of the sets shown in Fig. P22.57 is most acidic? Explain. PH HC CHCH C CH or CH,CH,CH2C CH Figure P22.57
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
A local university is considering changes to its class structure in an effort to increase professor productivity. The old schedule had each professor teaching 5 classes per week, with each class...
-
Golden Bear Construction Co. operates throughout California. The owner, Gaylan Beavers, employs 15 work crews. Construction supervisors report directly to Beavers, and the supervisors are trusted...
-
1. Visit a museum or gallery exhibition or attend a theater or musical performance. The activity (museum or performance) should have fun doing this. 2. Write a report that describes your experience....
-
Country risk refers to the ways governments restrict, or fail to restrict, business activities. The nature of such restrictions varies around the world. In each country, national economic success...
-
1. What types of decisions must Chad Thomas make daily for his companys operations to run effectively? Over the long run? 2. How did sales and marketing affect operations when they began to sell...
-
This is marketing DETAILS: Product Life Cycle 1. Introduction - It is the stage when the product is launched in the market. The company spends great amount of money to develop and introduce their...
-
When acetoacetic acid is decarboxylated in the presence of bromine, bromoacetone is isolated (see Fig. P22.68). The rate of appearance of bromoacetone is described by the following rate law: (The...
-
(a) Give the products that result from the ester hydrolysis of 1-cyclohexenyl acetate (compound A) below. (b) As the above data show, the G for hydrolysis of A is much more negative than the G for...
-
The quantity of CDs demanded by Michael Roux is twice the quantity that Adam Schmidt demands, whatever the price. Does that mean that Michael appreciates music more than Adam? Why or why not?
-
What is Computer Programming and How to Become a Computer Programmer?
-
What Do Programmers Do All Day?
-
How Do You Become a Computer Programmer?
-
Introduction to Accounting - Meaning, Objectives Fundamentals of Accounting
-
The domain of chemical reaction engineering consists of all chemical transformations (and that includes biological) of starting materials, derived from non-renewable and renewable resources, into a...
-
Discuss convergence of Gauss-Seidel iteration for the system 5x + 7y + 6z + 5w = 23 7x + 10y + 8z + 7w = 32 6x + 8y + l0z + 9w = 33 5x + 7y + 9z + 10w = 31.
-
Ask students to outline the reasons why the various elements of culture (social structures and control systems, language and aesthetics, religion and other belief systems, educational systems, etc.)...
-
Propose a mechanism that explains the following transformation. H2SO4 OH
-
Triethylamine, (C2H5)3N, like all amines, has a nitrogen atom with an unshared pair of electrons. Dichlorocarbene also has an unshared pair of electrons. Both can be represented as shown below. Draw...
-
The following order of reactivity is observed when the following alkenes are subjected to acid-catalyzed hydration: (CH3)2C==CH2 > CH3CH==CH2 > CH2==CH2 Explain this order of reactivity.
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


