Within each set, which compound should be more reactive in carbonyl-addition reactions? Explain your choices. (a) (c)
Question:
Within each set, which compound should be more reactive in carbonyl-addition reactions? Explain your choices.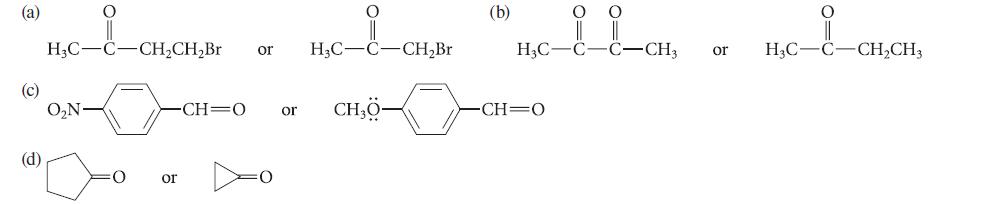
Transcribed Image Text:
(a) (c) (d) O H₂C-C-CH₂CH₂Br O₂N or or H₂C- -CH=0 or CH₂0- -CH₂Br (b) O CH=O O H₂C-C-C-CH, or H3C-C-CH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
We use the same principles to predict reactivity that we use to predict relative equilibrium constan...View the full answer

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Within set, which compound should be more reactive in carbonyl-addition reactions? Explain your choices. H,CCC CH or HCC CH2CH --
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The Grignard reaction is one of the classic methods in organic chemistry for the formation of C-C bonds. The two-step sequence involves initial reaction of an organohalogen compound with magnesium in...
-
What conditions must be met for revenue to be recorded? Can pledges meet those conditions?
-
Nibbles Cookies uses the accrual method of accounting and properly records transactions on the date they occur. Descriptions of customer transactions follows: a. Received $4,800 cash from customer...
-
Metropolis Country Club purchased a new tractor to be used for golf course maintenance. The tractor cost $64,200. Metropolis borrowed the purchase price from its bank on a 1-year, 7% note payable....
-
Customers use a variety of methods to provide feedback to companies about their experiences. Planetfeedback.com was developed as one such venue. Visit its website (www.planetfeedback.com) and...
-
The general ledger of Stephens Products, Inc. contains the following control account: If the materials charged to the one uncompleted job still in process amounted to $3,400, what amount of labor and...
-
Splish Clothiers is a small company that manufactures tall-mens suits. The company has used a standard cost accounting system. In May 2020, 10,600 suits were produced. The following standard and...
-
Write a curved-arrow mechanism for (a) The acid-catalyzed addition of methanol to benzaldehyde. (b) The methoxide-catalyzed addition of methanol to benzaldehyde.
-
Use resonance arguments to explain why (a) P-methoxybenzaldehyde is more basic than p-nitrobenzaldehyde. (b) 3-buten-2-one is more basic than 2-butanone.
-
Repeat Exercise 5 for predicting number of species caught from the surface temperature. Use the data in nybight.dat for this problem. Using the 1974 data, estimate the coefficients in a straight line...
-
At 3 1 st March, 2 0 2 3 , AB Ltd , had an Authorized Capital of K 3 5 , 0 0 0 divided into 1 0 , 0 0 0 7 . 5 % noncumulative per share being due on 3 0 th June, 1 9 6 4 . per share paid, the...
-
A Leadership and Workforce Development Perspective. The literature review should discuss the related literature, organized by topic or themes (not a list of sources). A literature review includes...
-
Critical Success Factors (CSF) are elements that are necessary for an organization or a project to attain its objectives. For example, Chief Executive support is a CSF for corporate sustainability...
-
Ultra Ceramic Products presented the following data for its operations for the month of October, 2020: Dept 1 Work in process, July t. 1(Conversion costs, 60%) 7,000 units Transferred to Dept 2 Work...
-
Choose a global organizational leader who demonstrated how a high level of ethical communication via social media technologies have worked best at building trust with virtual stakeholders. Identify a...
-
Using the set-up in the proof of Theorem 8.20, explain why Then prove that bjj = bji, justifying the statement that the restriction of A to V¥ is represented by a symmetric matrix. -1 Ayy - y J,...
-
You have accepted the engagement of auditing the financial statements of the C. Reis Company, a small manufacturing firm that has been your auditee for several years. Because you were busy writing...
-
Show how you could employ enamines in syntheses of the following compounds: (a) (b) (c) (d) O C OEt
-
Rank the following in order of increasing acidity for the indicated hydrogen atoms (bold) (1 = least acidic; 4 = most acidic). (a) (b) (c) O C OCH3 . CH3 H3C
-
Treating a solution of cis-1-decalone with base causes an isomerization to take place. When the system reaches equilibrium, the solution is found to contain about 95% trans-1-decalone and about 5%...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


