Write the two half-reactions that correspond to the oxidation of iodide ion in Eq. 16.36. HO +
Question:
Write the two half-reactions that correspond to the oxidation of iodide ion in Eq. 16.36.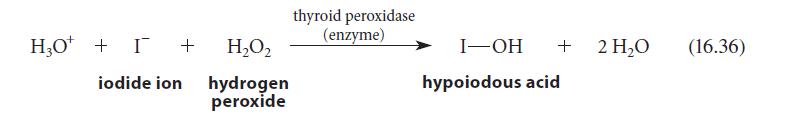
Transcribed Image Text:
H₂O + I + H₂O₂ iodide ion hydrogen peroxide thyroid peroxidase (enzyme) I-OH + hypoiodous acid 2 H₂O (16.36)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
In this reaction iodide ion I is oxidized by hydrogen ...View the full answer

Answered By

Ayush Mishra
I am a certified online tutor, with more than 3 years of experience in online tutoring. My tutoring subjects include: Physics, Mathematics and Mechanical engineering. I have also been awarded as best tutor for year 2019 in my previous organisation. Being a Mechanical Engineer, I love to tell the application of the concepts of science and mathematics in the real world. This help students to develop interest and makes learning fun and easy. This in turn, automatically improves their grades in the subject. I teach students to get prepared for college entry level exam. I also use to teach undergraduate students and guide them through their career aim.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write reactions that correspond to the following enthalpy changes: a. Hof for solid aluminum oxide b. the standard enthalpy of combustion of liquid ethanol [C2H5OH(l)]
-
The oxidation of iodide ion by the hypochlorite ion in the presence of hydroxide ions was studied at 25C, and the following initial rates data (Y. Chia and R. E. Connick, Journal of Physical...
-
The oxidation of iodide ion by arsenic acid, H3AsO4, is described by the balanced equation (a) if ? ?[I] / ?t = 4.8 ? 104 M/s, what is the value of ? [13] / ?f during the same time interval? (b) What...
-
People who earn a higher salary can afford more goods, including health care. However, according to Grossman, they will choose a higher desired health stock. Why is this so, according to the model?
-
Correcting errors in income statement transactions Broyo Corporation (Brow), a large paper company, reported the following income statement for its year ended December 31, 2008. Broyo applies IFRS...
-
The electric potential at the dot in FIGURE EX25.32 is 3140 V. What is charge q? -5.0 nC 2.0 cm 4.0 cmi 5.0 nC FIGURE EX25.32
-
Find the probability of selecting a number less than 1000.
-
A stream of ethylene glycol vapor at its normal boiling point and 1 atm flowing at a rate of 175 kg/mm is to be condensed at constant pressure. The product stream from the condenser is liquid glycol...
-
60. Which of the following is NOT a possible penalty for violation of the Clean Air Act? a. $25,000 per day, up to 1-year imprisonment, or both b. 15 years for willful or repeat violations $10,000...
-
The iodination reaction discussed in this section can be carried out on the amino acid tyrosine and related compounds in the laboratory with iodide ion in the presence of the enzyme thyroid oxidase....
-
Which should be faster: bromination of benzene or bromination of N,N-dimethylaniline? Explain your answer carefully. -N(CH3)2 N,N-dimethylaniline
-
Two identical spaceships approach an inertial observer \(\mathcal{O}\) at equal speeds but from opposite directions. A second observer \(\mathcal{O}^{\prime}\) traveling on one of the spaceships...
-
speed of the three phase motor does not vary greatly from the experiment. 1. Draw the symbol for a Three Phase Electric Motor. (Hint: remember the symbol table from the beginning of the semester?) 2....
-
Lifetime Insurance Company has two supporting departments (actuarial and premium), and two production departments (advertising and sales). Data from operations for the current year are as follows:...
-
Consider a wireless local area network (LAN) with an access point and 10 stations (Station 1, Station 2, Station 3, , and Station 10). Distributed coordination function (DCF), which is based on...
-
A worker needs to pump water from a reservoir to a big container that is open to the atmosphere. The water velocity at the surface of the reservoir is 2.5 m/s. The worker uses a 35-m long, 18-cm...
-
Identify each fringe benefit provided to Maggie and determine whether an exemption applies. (6 marks) Question 2: Explain the impact the fringe benefits will have on Maggie's taxable income and/or...
-
What is the distinction between a capital expenditure and a revenue expenditure?
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
Which should be faster: bromination of benzene or bromination of N.N-dimethvlaniline? Explain your answer carefully. N,N-dimethylaniline
-
Predict the predominant product(s) from: Mononitration of m-bromoiodobenzene
-
In each of the following sets, rank the compounds in order of increasing harshness of the reaction conditions required to accomplish the indicated reaction. Friedel-Crafts acylation of chlorobenzene,...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


