Which should be faster: bromination of benzene or bromination of N,N-dimethylaniline? Explain your answer carefully. -N(CH3)2 N,N-dimethylaniline
Question:
Which should be faster: bromination of benzene or bromination of N,N-dimethylaniline? Explain your answer carefully.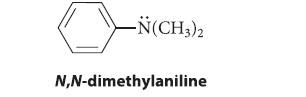
Transcribed Image Text:
-N(CH3)2 N,N-dimethylaniline
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Bromination of NNdimethylaniline is faster because nitrog...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which should be faster: bromination of benzene or bromination of N.N-dimethvlaniline? Explain your answer carefully. N,N-dimethylaniline
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
A company with no cash, no assets in place and no debt has two mutually exclusive projects: A and B. Both projects require investment of $100 million and last for one year. Project A yields $110...
-
If any fi rms price of labor and capital each double, what will happen to the expansion path (i.e., locus of tangencies between the isoquants and isocost curves)? What will happen to the fi rms...
-
Income statement formats Information from GoodLuck Brands's income statements for the years ended December 31, 2008, 2007, and 2006, is shown next. GoodLuck Brands is a U.S.-based manufacturer and...
-
Two point charges q a and q b are located on the x-axis at x = a and x = b. FIGURE EX25.34 is a graph of V, the electric potential. a. What are the signs of q a and q b ?b. What is the ratio |q a /q...
-
Find the probability of selecting a number greater than 1000.
-
Refer to the accompanying screen display. a. Express the confidence interval in the format that uses the "less than" symbol. Given that the original listed data use one decimal place, round the...
-
stuck Required information [The following information applies to the questions displayed below.] The following transactions pertain to Accounting Solutions Inc. Assume the transactions for the...
-
Write the two half-reactions that correspond to the oxidation of iodide ion in Eq. 16.36. HO + I + HO iodide ion hydrogen peroxide thyroid peroxidase (enzyme) I-OH + hypoiodous acid 2 HO (16.36)
-
Explain why the nitration of anisole is much faster than the nitration of thioanisole under the same conditions. -CH3 anisole -SCH3 thioanisole
-
The three-month interest rate in both the US and the UK is 12% in the respective money market conventions. Suppose the three-month period has 92 days. The spot exchange rate is 1 = $1.80. What is the...
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
The following expenditures were incurred in connection with a new machine acquired by a metals manufacturing company. Identify those that should be included in the cost of the asset. (a) Freight...
-
How do network effects help Facebook fend off smaller social-networking rivals? Could an online retailer doing half as much business compete on an equal footing with Amazon in terms of costs? Explain.
-
Using benzene and any other reagents, outline a synthesis of each of the following compounds. Tert-butylcyclohexane
-
Give the products expected (if any) when nitrobenzene reacts under the following conditions. (a) Cl2, FeCl3, heat (b) Fuming HNO3, H2SO4 (c) H3C-C-CI, AICI,( 1.1 equiv.), then H2O
-
Explain how you would distinguish between ethylbenzene, p-xylene, and styrene solely by NMR spectroscopy.
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


