Explain why the nitration of anisole is much faster than the nitration of thioanisole under the same
Question:
Explain why the nitration of anisole is much faster than the nitration of thioanisole under the same conditions.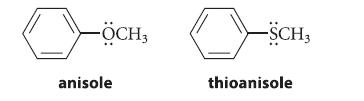
Transcribed Image Text:
-ÖCH3 anisole -SCH3 thioanisole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Although both the CH 3 O and the CH 3 S groups are activating ort...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid, p-methoxybenzoic acid or p-(methylthio) benzoic acid?Explain. Anisole (CGH5OCH3)...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Let A be an invertible n n matrix, and let B be an n p matrix. Explain why A -1 B can be computed by row reduction: If A is larger than 2 x 2, then row reduction of [A B] is much faster than...
-
A W. Hopes year ended on 30 June 2011. Write up the ledger accounts, showing the transfers to the financial statements and the balances carried down to the next year for the following: (a)...
-
Income statement formats Information from Cemex. S.A.B.'s income statements for the years ended December 31, 2006 and 2007, is shown in the following display. Cemex is a Mexican construction firm...
-
Two point charges 2.0 cm apart have an electric potential energy -180 J. The total charge is 30 nC. What are the two charges?
-
Find the probability of selecting a number divisible by 1000.
-
A solar collector consists of a parallel plate channel that is connected to a water storage plenum at the bottom and to a heat sink at the top. The channel is inclined 0 = 30 from the vertical and...
-
E. $ 2.30 17 Genesis Scents has two divisions: the Cologne Division and the Bottie Division. The Bottle Division produces containers that can be used by the Cologne Division. The Bottle Division's...
-
Which should be faster: bromination of benzene or bromination of N,N-dimethylaniline? Explain your answer carefully. -N(CH3)2 N,N-dimethylaniline
-
Biphenyl (phenylbenzene) undergoes the FriedelCrafts acylation reaction, as shown by the following example. (a) On the basis of this result, what is the directing effect of the phenyl group? (b)...
-
Reconsider the axial stiffness data given in Exercise 8. ANOVA output from Minitab follows: Pooled StDev = 32.39. Tukey's pair-wise comparisons Family error rate = 0.0500 Individual error rate =...
-
Below are incomplete financial statements for Hurricane, Incorporated Required: Calculate the missing amounts. Complete this question by entering your answers in the tabs below. Income Statement Stmt...
-
TBTF Incorporated purchased equipment on May 1, 2021. The company depreciates its equipment using the double-declining balance method. Other information pertaining to the equipment purchased by TBTF...
-
Coco Ltd. manufactures milk and dark chocolate blocks. Below is the information relating to each type of chocolate. Milk Chocolate Selling price per unit $6 Variable cost per unit $3 Sales mix 4 Dark...
-
Data related to 2018 operations for Constaga Products, a manufacturer of sewing machines: Sales volume 5,000 units Sales price $300.00 per unit Variable production costs Direct materials 75.00 per...
-
6. (20 points) Sections 3.1-3.5, 3.7 Differentiate the following functions, state the regions where the functions are analytic. a. cos(e*) b. 1 ez +1 c. Log (z+1) (Hint: To find where it is analytic,...
-
Several years ago Walker Security purchased for $120,000 a well-known trademark for padlocks and other security products. After using the trademark for three years, Walker Security discontinued it...
-
Identify the most stable compound:
-
Explain why The methyl group in the following compound has an unusual chemical shift of (- 1.61), about 4 ppm lower than the chemical shift of a typical allylic methyl group. : Na sodium salt of...
-
Within each set, which compound should show NMR absorptions with the greater chemical shifts? Explain your choices. (1) (2)
-
Outline laboratory syntheses of each of the following compounds, starting with benzene and any other reagents. (The references to equations will assist you with nomenclature.) (a) p-dibromobenzene...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


