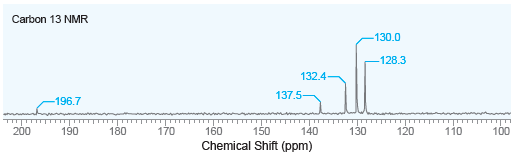
A compound with molecular formula C 13 H 10 O produces a strong signal at 1660 cm
Question:
Transcribed Image Text:
Carbon 13 NMR -130.0 -128.3 132.4- 137.5- -196.7 200 180 170 160 150 Chemical Shift (ppm) 140 130 120 110 100 190
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)

Answered By

Mario Alvarez
I teach Statistics and Probability for students of my university ( Univerisity Centroamerican Jose Simeon Canas) in my free time and when students ask for me, I prepare and teach students that are in courses of Statistics and Probability. Also I teach students of the University Francisco Gavidia and Universidad of El Salvador that need help in some topics about Statistics, Probability, Math, Calculus. I love teaching Statistics and Probability! Why me?
** I have experience in Statistics and Probability topics for middle school, high school and university.
** I always want to share my knowledge with my students and have a great relationship with them.
** I have experience working with students online.
** I am very patient with my students and highly committed with them
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound with molecular formula C 9 H 10 O exhibits the following spectra ( 1 H NMR, 13 C NMR, and IR). Identify the structure of this compound.. Proton NMR 10 Chemical Shift (ppm) Carbon NMR 128.5...
-
The IR spectrum of a compound with molecular formula C5H8O was obtained in CCl4 and is shown in Figure 13.42. Identify the compound. Wavelenga qum) 15 16 14 3600 340) 3800 3300 3000 280K 2600 2400...
-
A compound with molecular formula C 8 H 10 O produces six signals in its 13 C NMR spectrum and exhibits the following 1 H NMR spectrum. Deduce the structure of the compound. Proton NMR Chemical Shift...
-
The table shows the fees for refund anticipation loans (RALs) offered by an online tax preparation firm. Find the annual rate of interest for each of the following loans. Assume a 360-day year. (A) A...
-
Pete is considering placing a bet on the NCAA playoff game between Indiana and Purdue. Without any further information, he believes that each team has an equal chance to win. If he wins the bet, he...
-
Use three repetitions of the NewtonRaphson algorithm to approximate the following: 3 6
-
6. Because proprietary funds are accounted for in much the same manner as commercial business organizations, is it appropriate for FASB pronouncements to be used for their accounting?
-
Develop an exponential smoothing forecast (a = 0.3) for February 2012 through January 2013. Assume that your forecast for January 2012 was 100. Calculate the MFE, MAD, and MAPE values for February...
-
May Co. is planning to sell 900 boxes of ceramic tile, with production estimated at 870 boxes during June. Each box of tile requires 44 pounds of clay mix and a quarter hour of direct labor. Clay mix...
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
A compound with molecular formula C 9 H 10 O exhibits a strong signal at 1687 cm 1 in its IR spectrum. The 1 H and 13 C NMR spectra for this compound are shown below. Identify the structure of this...
-
A ketone with molecular formula C 9 H 18 O exhibits only one signal in its 1 H NMR spectrum. Provide a systematic (IUPAC) name for this compound.
-
Bowler Company is considering purchasing a packaging machine for $20,000 that will have a residual value of $100 after 10 years. It would be depreciated using the straightline method. The machine...
-
1. The following data are available for JURIS DOCTOR CORP: Purchased raw materials from supplier amounting to P 40,000 on account.; During the month, raw materials costing P 30,000 were issued to...
-
The following financial information is available for Concord Corporation. (in millions) 2025 2024 Average common stockholders' equity $2,500 $2,600 Dividends declared for common stockholders 305 594...
-
Vecton's Bakery manufactures apple turnovers that passes through 4 sequential processes. Production data for February for Department 4 of the operation is as follows: Production data Units Opening...
-
write a code in java where we apply the sets and subsets to obtain functions as results. Let A= {1,2,3,4}, B={5,6,7,0}, C={8,9,10,11} and f: AB g:BC h: BC, all function are 1 to 1 a) Form the...
-
Consider the following LC-3 program. .ORIG x3000 LEA R1, LABEL LDR RO, R1, #231 LDI R1, LOCAL AND R3, R3, #0 LOOP AND R2, RO, R1 BRZ SHIFT ADD R3, R3, #1 SHIFT ADD R1, R1, R1 BRnp LOOP HALT LABEL...
-
Solve each system. 4.x - 3 -y + 3 1 x+ 3 1 2 2 2 = -6 3 2 = 0 = 2 = -5
-
Recall that Chapter 8 described the binary search algorithm for finding a particular entry in an ordered list. The idea behind binary search is to begin looking in the exact center of the list. If...
-
Rank the following alcohols in order of increasing ease of acid-catalyzed dehydration. OH OH OH
-
Acid-catalyzed dehydration of neopentyl alcohol, (CH3)3CCH2OH, yields 2-methyl-2- butene as the major product. Outline a mechanism showing all steps in its formation.
-
Acid-catalyzed dehydration of either 2-methyl-1-butanol or 3-methyl-1-butanol gives 2-methyl-2-butene as the major product. Write plausible mechanisms that explain these results.
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


