Consider the following reaction. Predict whether an increase in temperature will favor reactants or products. Justify your
Question:
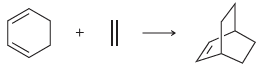
Transcribed Image Text:
+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (18 reviews)
Recall that G has two components H and TS We must analyze each term separately The first term is exp...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction to produce methyl acetate: When this reaction is carried out with CH3OH containing radioactive oxygen-18, the water produced is not radioactive. Explain. CH,OH CH...
-
Consider the following reaction at some temperature: H2O(g) + CO(g) H2(g) + CO2(g) K = 2.0 Some molecules of H2O and CO are placed in a 1.0- L container as shown below. When equilibrium is reached,...
-
Consider the following reaction at 248oC and 1.00 atm: CH3Cl(g) + H2(g) CH4(g) + HCl(g) For this reaction, the enthalpy change at 248oC is 283.3 kJ/ mol. At constant pressure the molar heat...
-
Suppose that one worker can shovel snow from a storefront sidewalk in 50 minutes and another worker can shovel it in 30 minutes. How long will it take if they work together?
-
1. Develop and submit the Mini-Case Just Right Globalization 2. Is there more to the "not-invented-here" syndrome than simply hurt feelings on the part of those who believe they are being dictated to...
-
Find the first four terms of the indicated expansions by use of the binomial series. 1 + x 2
-
The following annual charges are incurred in respect of a machine in a shop where manual labour is almost nil and work is done by means of five machines of exactly same type of specification. (i)...
-
Lavoie Corp acquired new equipment at a cost of $100000 plus 7% provincial sales tax and 5% GST (GST is a recoverable tax). The company paid $1700 to transport the equipment to its plant. The site...
-
Exercise 20-33A Merchandising: Budgeted balance sheet LO P3 The following information is available for Zetrov Company: a. The cash budget for March shows an ending bank loan of $19,000 and an ending...
-
Assume that the taxpayers, George A. Warden (social security number 333-33-3330) and Mary S. Warden (social security number 444-44-4440) file a joint return. Both are 50-years old, have good...
-
In the presence of a special type of catalyst, hydrogen gas will add across a triple bond to produce a double bond: The process is exothermic. Do you expect a high temperature to favor products or...
-
When an amine is protonated, the resulting ammonium ion is not electrophilic: However, when an imine is protonated, the resulting iminium ion is highly electrophilic: Explain this difference in...
-
Heitger Company is a job-order costing firm that uses activity-based costing to apply overhead to jobs. Heitger identified three overhead activities and related drivers. Budgeted information for the...
-
What is brand awareness for Jam & Daisies ? their leaning advantage, consideration advantage, choice advantages? 5. what is the recommendation of brand awareness? 6. What is Brand recognition? 7....
-
On August 1st, Custom Car Co's work in process inventory was $24900; its raw materials inventory was $6000; manufacturing overhead had a $1800 debit balance. Work in Process Subsidiary Data 8/1:...
-
Case: Castoro & Partners, CPAs is auditing Cloud 9 for the FY2023. Cloud 9 is a small public company and has been an audit client of Castoro & Partners since 2018. Materiality Methodology: Overall...
-
1)Solve the following differential equations by Undetermined Coefficient Method. dy dx dy - 4- 4+ 4y = 16x2e2x dx
-
Every year Monty Industries manufactures 8,600 units of part 231 for use in its production cycle. The per unit costs of part 231 are as follows: Direct materials Direct labor Variable manufacturing...
-
Use positive exponents to rewrite. Vx. Vx
-
A certain Christmas tree ornament is a silver sphere having a diameter of 8.50 cm. Determine an object location for which the size of the reflected image is three-fourths the size of the object. Use...
-
If we examine Table 21.1, we find that the methylphenols (cresols) are less acidic than phenol itself. For example, This behavior is characteristic of phenols bearing electron-releasing groups....
-
When o-chlorotoluene is subjected to the conditions used in the Dow process (i.e., aqueous NaOH at 350oC at high pressure), the products of the reaction are o-cresol and m-cresol. What does this...
-
When 2-bromo-1, 3-dimethylbenzene is treated with sodium amide in liquid ammonia, no substitution takes place. This result can be interpreted as providing evidence for the elimination-addition...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


