In the presence of a special type of catalyst, hydrogen gas will add across a triple bond
Question:
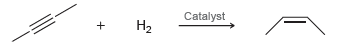
The process is exothermic. Do you expect a high temperature to favor products or reactants?
Transcribed Image Text:
H2 Catalyst
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
In order to determine if reactants or products are favored at high temperature we must cons...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The synthesis of ethyl chloride is accomplished by reacting ethylene with hydrogen chloride in the presence of an aluminum chloride catalyst: C 2 H 4 (g) + HC1 (g) catalyst ? C 2 H 5 C1 (g): ?H ?...
-
Caryophyllene, a compound found in oil of cloves, has the molecular formula C15H24 and has no triple bonds. Reaction of caryophyllene with an excess of hydrogen in the presence of a platinum catalyst...
-
When ethene is mixed with hydrogen in the presence of a platinum catalyst, hydrogen adds across the double bond to form ethane. At room temperature, the reaction goes to completion. Predict the signs...
-
Due to acid rain, the percentage of lakes in Scandinavia that lost their population of brown trout increased dramatically between 1940 and 1975. Based on a sample of 2850 lakes, this percentage can...
-
In terms of ICS/SCADA risk management and auditing, demonstrate how to use your knowledge of the risk as function of M, AV, T, and V. That is R = f (M, AV, T, V). Where: R - Risk, M - Mission...
-
Find the indicated quantities for the appropriate arithmetic sequence. In order to prevent an electric current surge in a circuit, the resistance R in the circuit is stepped down by 4.0 after each...
-
Calculate the MHR for the recovery of overheads for a group of three machines from the following data: Original cost of 3 machines Rs 56,800 Depreciation at 10% per annum (straight line method)...
-
A market research firm used a sample of individuals to rate the purchase potential of a particular product before and after the individuals saw a new television commercial about the product. The...
-
actice Assignment Gradebook ORION Downloadable eTextbook gnment Intermediate Accounting (ACC 615 CALCIATOS FESTEN UNTER VERSION BACK NEX Exercise 18-10 Vaughn Windows mad ures and sells custom storm...
-
Thornby Inc. has completed its fiscal year on December 31. The auditor, Kim Holmes, has approached the CFO, Brad Potter, regarding the year-end receivables and inventory levels of Thornby Inc. The...
-
Identify whether each of the following factors will affect the rate of a reaction: (a) K eq (b) G (c) Temperature (d) H (e) E a (f ) S
-
Consider the following reaction. Predict whether an increase in temperature will favor reactants or products. Justify your prediction. +
-
Explain why it is common to verify total officers compensation even when the tests of controls and substantive tests of transactions results in payroll are excellent. What audit procedures can be...
-
Your company has a Microsoft 365 E5 subscription. You need to review the Advanced Analysis tab on emails detected by Microsoft Defender for Office 365. What type of threat policy should you...
-
(a) The Bright company is evaluating a project which will cost Rs 1,00,000 and will have no salvage value at the end of its 5-year life. The project will save costs of Rs. 40,000 a year. The company...
-
Dispatcher Collins is retiring after 30 years on the job. If each of the 38 officers in the department contributes $9 for a retirement gift, what is the total amount that could be spent on this gift
-
XYZ CO Adjusted Trial Balance Debit Credit Cash Accounts receivable Office supplies Prepaid rent $ 40 850 1 490 1 530 4 000 Office equipment Accumulated Depreciation Accounts payable 7 000 $ 450 1...
-
What positive outcomes could result from implementing job enlargement, job rotation, and job enrichment in an organization with which you are familiar? What objections or obstacles might be...
-
Find all real solutions. Check your results. 4 x - 3x = 1 x - 9
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Given its mission of providing information to the world, should Google censor searches in China?
-
Using a different method for each part, but taking care in each case to select a good method, show how each of the following transformations might be accomplished: (a) (b) (c) (d) (e) NH2 NH2 CH3O...
-
Review the chemistry of amines given in earlier sections and provide a specific example for each of the previously illustrated reactions.
-
Para-nitrosation of N,N-dimethylaniline (C-nitrosation) is believed to take place through an electrophilic attack by N+O ions. (a) Show how N+O ions might be formed in an aqueous solution of NaNO 2...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


