Draw the cyclic hemiacetal that is formed when each of the following bifunctional compounds is treated with
Question:
(a)
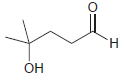
(b)
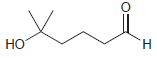
(c)
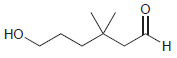
(d)
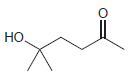
Transcribed Image Text:
н Он Но H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
a...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For questions 6 - 7, answer the questions about similar right triangles. 6. Given that AABC - ADEC, find the value of x. 7. Given that AABC - ADEC, find the value of x. 30 18 A 2x-1 X+1 D 6x-2 2x+8 A...
-
Draw the enolate ion that is formed when each of the following compounds is treated with sodium ethoxide. In each case, draw all resonance structures of the enolate ion, and predict whether a...
-
Draw the product formed when each of the following compounds is treated with NaNO 2 and HCl: (a) (b) NH2 N.
-
When component Auditors are involved in the audit of group financial statements the group Auditors are required to a. consider the independence and professional reputation of the component Auditors...
-
The Oakman Company (see Short Exercise S24-1) has refined its allocation system by separating manufacturing overhead costs into two cost pools-one for each department. The estimated costs for the...
-
Redo Problem 13.47 using Aspen Plus. Problem 13.47 The reaction is used as a step in the process to convert waste sulfur dioxide to sulfuric acid. Starting with stoichiometric amounts of sulfur...
-
What percent of U.S. adults ages 25 and over (a) have a degree and are unemployed? (b) have some college education, but no degree, and are not in the labor force? (c) are employed and high school...
-
Browne and Red, both C corporations, formed the BR Partnership on January 1, 2013. Neither Browne nor Red is a personal service corporation, and BR is not a tax shelter. BR's gross receipts were $4.6...
-
Armadale company manufactures and sells three video game consoles The Alpha X. the Beta Y and the Gammame systems Information for each product is shown below 4 S Alpha 30.0015 5,000 Beta 50.00 Gamma...
-
A chemical reaction has a theoretical yield of 19.98 g and a percent yield of 88.40%. What is the actual yield?
-
Which of the following terms best describes the relationship between d-fructose and d-glucose? Explain your choice. (a) Enantiomers (b) Diastereomers (c) Constitutional isomers
-
Identify the hydroxyaldehyde that will cyclize under acidic conditions to give the following hemiacetal: -OH
-
Suppose there are \(n\) stocks. Each of them has a price that is governed by geometric Brownian motion. Each has \(v_{i}=15 \%\) and \(\sigma_{i}=40 \%\). However, these stocks are correlated, and...
-
Exercise 11-5 Profit allocation in a partnership LO3 Dallas and Weiss formed a partnership to manage rental properties, by investing $198,000 and $242,000, respectively. During its first year, the...
-
Reading following articles and answer the questions: https://www.afr.com/technology/ai-is-coming-for-white-collar-jobs-gates-warns-20230123-p5cev7...
-
1. Citing an example in each case, briefly explain four types of book keeping errors which are not disclosed by trial balance 2. The trial balance extracted from the books of james as at 30 september...
-
Use the universal gravitation formula to determine which object has a larger effect on the Earth's motion through space: the Sun or the Moon. Explain how you are determining this, including very...
-
Pro Cycling Shop is a medium-size seller of the high-end bicycle. Since starting the company 15 years ago, Pro Cycling Shop has been a competitive company across Sarawak, Brunei, Kalimantan, and...
-
In Exercises 52 through 56, you need to know that a cylinder of radius r and height h has volume V = r 2 h and lateral (side) surface area S = 2rh. A circular disk of radius r has area A = r 2 . A...
-
A police officer pulls you over and asks to search your vehicle because he suspects you have illegal drugs inside your car. Since he doesn't have reasonable suspicion to search your car, legally he...
-
Determine whether the following objects are chiral or achiral. T 0/
-
If you had the two enantiomers of carvone in unmarked bottles, could you use just your nose and a polarimeter to determine? (a) Whether it is the (+) or (-) enantiomer that smells like spearmint? (b)...
-
When optically pure (R)-2-bromobutane is heated with water, butan-2-ol is the product. The reaction forms twice as much (S)-butan-2-ol as (R)-butan-2-ol. Calculate the e.e. and the specific rotation...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...
-
On an average day, a company writes checks totaling $1,500. These checks take 7 days to clear. The company receives checks totaling $1,800. These checks take 4 days to clear. The cost of debt is 9%....
-
Olds Company declares Chapter 7 bankruptcy. The following are the book values of the asset and liability accounts at that time. A bankruptcy expert estimates that administrative expense will total $...

Study smarter with the SolutionInn App


