Identify the hydroxyaldehyde that will cyclize under acidic conditions to give the following hemiacetal: -OH
Question:
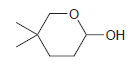
Transcribed Image Text:
-OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)

Answered By

RADHIKA MEENAKAR
I am a qualified indian Company Secretary along with Masters in finance with over 6 plus years of professional experience. Apart from this i am a certified accounts and finance tutor on many online platforms.
My Linkedin profile link is here https://www.linkedin.com/in/radhika-meenakar-88b9808a/
5.00+
12+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When ethylene oxide is treated with a strong nucleophile, the epoxide ring is opened to form an alkoxide ion that can function as a nucleophile to attack another molecule of ethylene oxide. This...
-
The mechanism for acidic hydrolysis of a nitrile resembles the basic hydrolysis, except that the nitrile is first protonated, activating it toward attack by a weak nucleophile (water). Under acidic...
-
Consider the three constitutional isomers of dioxane (C 4 H 8 O 2 ): One of these constitutional isomers is stable under basic conditions as well as mildly acidic conditions and is therefore used as...
-
Krell Industries has a share price of $22 46 today. If Krell is expected to pay a dividend of S0.83 this year, and its stock price is expected to grow to $24.11 at the end of the year, what is...
-
Eason Company manufactures wheel rims. The controller expects the following ABC allocation rates for 2018: Eason produces two wheel rim models: standard and deluxe. Expected data for 2018 are as...
-
How does trading differ from speculating?
-
Explain why you cannot perform the chi-square independence test on these data. Conditional Relative Frequencies In Exercises 32-39, use the contingency table from Exercises 29-31, and the following...
-
How would the court most likely rule on Scotts employee status? James Blatt hired Marilyn Scott to sell insurance for the Massachusetts Mutual Life Insurance Co. Their contract stated, Nothing in...
-
A local bookstore is considering adding a coffee shop to their store. Building the coffee shop will cost $256,501.00 today. The bookstore is going to try this project for five years. The bookstore...
-
Based on a careful work study in the Hofstetter crop, the result shown in the following table have been observed: a) Compute the normal time for each work element. b) If the allowance for this type...
-
Draw the cyclic hemiacetal that is formed when each of the following bifunctional compounds is treated with aqueous acid: (a) (b) (c) (d) H.
-
The following compound has one aldehyde group and two OH groups: Under acidic conditions, either one of the OH groups can function as a nucleophile and attack the carbonyl group, giving rise to two...
-
Can the Bank of Korea achieve price stability and economic growth simultaneously? The Bank of Koreas monetary policy is to reduce the vulnerability of South Korean won and achieve price stabilization...
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
Charlene wrote a letter to Rachel offering to sell her car, a Proton Saga, for RM 60,000. The letter reached Rachel on 25. 11.2020. Rachel sent her letter of acceptance at 3 p.m. on the same day....
-
Data for the risk premium sensitivities (b, s, and h) as well as the beta coefficient for the CAPM of two companies are listed in the following table: Company b s h ERP SMBP HMLP Beta Alpha 1.1114...
-
Free-Response Questions 1. m Initial position eviribrA ARAL m Incline raised to 0 <0max pr A block of mass m is initially at rest on a rough board, which is initially horizontal on a tabletop. The...
-
A picture frame sits atop a bookshelf. When the bookshelf is bumped, the frame tumbles to the floor, landing after 0.64 s. How tall is the bookshelf?
-
Each ounce of Food I contains 3 g of carbohydrate and 2 g of protein, and each ounce of Food II contains 5 g of carbohydrate and 3 g of protein. Suppose x ounces of Food I are mixed with y ounces of...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
A chemist finds that the addition of (+)-epinephrine to the catalytic reduction of butan-2-one (Figure 5-16) gives a product that is slightly optically active, with a specific rotation of +0.45o...
-
1. Make a model of each compound, draw it in its most symmetric conformation, and determine whether it is capable of showing optical activity. (a) 1-bromo-1-chloroethane (b) 1-bromo-2-chloroethane...
-
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


