For each of the following reactions determine whether ÎS for the reaction (ÎS sys ) will be
Question:
a.
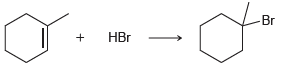
b.
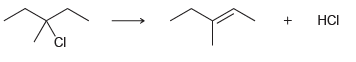
c.
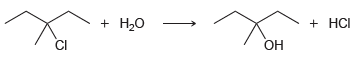
d.
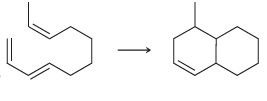
e.
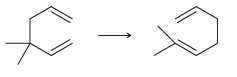
Transcribed Image Text:
Br НBr HCI CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (19 reviews)
a S sys is expected to be negative a decrease in entropy because ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) For each of the following reactions, predict the sign of ÎH° and ÎS° and discuss briefly how these factors determine the magnitude of K. (b) Based on your general chemical...
-
For each of the following reactions, give the curved-arrow notation and write the analogous Bonsted acid-base reaction. H,C-CH,-Br: + :C=N: H,C-CH-C=N: + :Br:
-
For each of the following reactions. provide the following information. (a) Give the structures of all products (including stereoisomers). (b) If more than one product is formed, give the...
-
You are looking at buying a piece of real estate and you intend to borrow as much as you possibly can from a bank to buy the property. The bank you are dealing with has a requirement that the LVR for...
-
Suppose you want to perform an experiment to verify the problems that can be caused by random insert/remove pairs. Here is a strategy that is not perfectly random, but close enough. You build a tree...
-
An online apparel retailer runs regular marketing campaigns on social media channels. The retailer is considering four social media marketing campaigns on Facebook, Instagram, Pinterest, and Twitter...
-
China is a huge, attractive market with growing affluence. Before exporting to China, most firms conduct market research to understand the Chinese market better. Two useful research sites are the...
-
John Smith has developed the following forecasting model: Y = 36 + 4.3X1 Where Y = Demand for K10 air conditioners X1 = the outside temperature (F) (a) Forecast the demand for K10 when the...
-
9. Co. Y has the following beginning inventory, purchases and ending inventory: Beginning inventory 300 units at $5 each, Purchase 6/1 300 units at $10 each, Purchase 9/1 300 units @ 15 each, and...
-
A major pharmaceutical wholesaler buys brand drugs from a manufacturer at wholesale prices and sells them to pharmacies at retail prices. It estimates that the wholesale (W) price, the retail (R)...
-
Explain: The United States can make certain toys with greater productive efficiency than can China. Yet we import these toys from China. Relate your answer to the ideas of Adam Smith and David...
-
The percentage that casinos make on the average bet is called the a. Vig. b. Rip. c. Take. d. Rob.
-
Show that the following eight vectors are pairwise orthogonal: \[\begin{aligned}& s 1=(1,1,0,0,0,0,0,0)^{T} \\& s 2=(0,0,1,1,0,0,0,0)^{T} \\& s 3=(0,0,0,0,1,1,0,0)^{T} \\& s 4=(0,0,,0,0,0,1,1)^{T}...
-
31. What is meant by path? 32. Give the formula for calculating D4 and D8 distance. 33. What is geometric transformation? 34. What is image translation and scaling? 35. Define the term Luminance
-
1. Explain Brightness adaptation and Discrimination 2.Explain sampling and quantization:
-
3. Explain about Mach band effect? 4. Explain color image fundamentals. 5. Explain CMY model.
-
1. Describe the fundamental steps in image processing? 2. Explain the basic Elements of digital image processing:
-
3. Explain the Structure of the Human eye 4. Explain the RGB model
-
Graph each polynomial function. Give the domain and range. f(x) = 2x + 3
-
The liquidliquid extractor in Figure 8.1 operates at 100F and a nominal pressure of 15 psia. For the feed and solvent flows shown, determine the number of equilibrium stages to extract 99.5% of the...
-
Which of the following alkyl halides would you expect to undergo Friedel-Crafts reaction without rearrangement? Explain. (a) CH3CH2Cl (b) CH3CH2CH (Cl) CH3 (c) CH3CH2CH2Cl (d) (CH3) CCH2Cl (e)...
-
What is the major mono-substitution product from the Friedel-Crafts reaction of benzene with 1-chloro-2-methylpropane in the presence of AlCl3?
-
Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each of the followingacylbenzenes: (b) (a)
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


