Identify the weakest C!H bond in each of the following compounds: (a) (b) (c) (d)
Question:
Identify the weakest C!H bond in each of the following compounds:
(a)
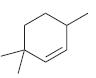
(b)
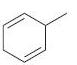
(c)
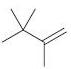
(d)
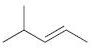
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
a...View the full answer

Answered By

Rustia Melrod
I am a retired teacher with 6 years of experience teaching various science subjects to high school students and undergraduate students. This background enables me to be able to help tutor students who are struggling with the science of business component of their education. Teaching difficult subjects has definitely taught me patience. There is no greater joy for me than to patiently guide a student to the correct answer. When a student has that "aha!" moment, all my efforts are worth it.
The Common Core standards are a useful yardstick for measuring how well students are doing. My students consistently met or exceeded the Common Core standards for science. I believe in working with each student's individual learning styles to help them understand the material. If students were struggling with a concept, I would figure out a different way to teach or apply that concept. I was voted Teacher of the Year six times in my career. I also won an award for Innovative Teaching Style at the 2011 National Teaching Conference.
4.90+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
Without using Fig. 13.3, predict which bond in each of the following groups is the most polar. a. COF, SiOF, GeOF b. POCl, SOCl c. SOF, SOCl, SOBr d. TiOCl, SiOCl, GeOCl e. COH, SiOH, SnOH f. AlOBr,...
-
Use the symbols δ+ and δ- to show the direction of polarity of the indicated bond in each of the following compounds (for example, a. HO--H b. F--Br c. H3C--NH2 d. H3C--Cl...
-
a. Over a two year time horizon an investor experiences the following net cash flows: Year 0 1 2 Cash flow -$8,000 $5,280 $5,320 What is the internal rate of return for the investor? Demonstrate that...
-
The contribution margin ratio of Thronson Corporation's only product is 61%. The company's monthly fixed expense is $456,300 and the company's monthly target profit is $42,300. Required: Determine...
-
Let R be the rectangle consisting of all points (x, y) such that 0 x 2, 2 y 3. Calculate the following double integrals. Interpret each as a volume. JJxy R xy dx dy
-
7. Should a company operate a diversified portfolio of businesses? What are the arguments for and against?
-
Kimball Company is a small retail business that uses a manual data processing system similar to the one described in the chapter. Among its special-purpose journals are multicolumn cash receipts and...
-
assignment3b question 8 + Assignment 3-B REQUIRED Question 8 of 9 2.11/5 View Policies Show Attempt History Current Attempt in Progress Grouper Architects incorporated as licensed architects on April...
-
a. What specific HR functions (recruiting, interviewing, and so on) can you identify Donald Trump using on the show? Make sure to give specific examples. b. What specific HR functions can you...
-
Prove that C V = - (U/V) T (V/T) U.
-
Calculate the single-factor and multiple-factor productivities.
-
Pat needs to purchase airplane tickets to fly home at the end of the term. The last exam schedule slot is Friday at 3 p.m., but Pats exams havent yet been scheduled. Exams are scheduled centrally at...
-
+ Given f(x) = x - 9 and g(x) = x+9, complete the following. (a) Find f(g(x)) and g(f(x)). (Simplify your answers completely.) f(g(x)) = g(f(x)) = (b) What does this tell us about the relationship...
-
Case Study - Rhonda Rhonda is a 28-year-old woman who has been referred to your agency by a local probation officer. Rhonda reported that she has "fired" three counselors in the past and most...
-
Calculating depreciationpartial periods LO2, 3 West Coast Tours runs boat tours along the west coast of British Columbia. On March 5, 2020, it purchased, with cash, a cruising boat for $936,000,...
-
Question 1. Write down the form of partial fractions needed to decompose the following: 482+2 (a) s32s24s 482+2 (c) s36s20 482 +2 - 4s8 (b) 8. 3 - 282 482+2 (d) s3 +2s2 - 2 Note: You are not being...
-
On December 31, 2022, Ace Hardware reported the following information on its balance sheet Accounts Receivable Allowance for Doubtful Accounts $900,000 $54,000 (credit) During 2023, the Company had...
-
Divide. 80r2 40r + 10 10r
-
How is use of the word consistent helpful in fraud reports?
-
Classify each of the following monosaccharide?s: la) 0 (c) (b) (d) CH- . H- C=0 C=0 -- -- -- -- -- -- -- -- -- H2OH -- CH- Ribulose CH Threose CH- Tagatose 2-Deoxyribose
-
Convert the following Fischer projections into tetrahedral representations, and assign R or S stereochemistry toeach: H (b) (a) H2N- - (c) - - CH-CH CH CH
-
Which of the following Fischer projections of glyceraldehydes represent the sameenantiomer? CH- - 3 - -CH- - - CH2 A
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


