Question: In this chapter, we have seen that an acetylide ion can function as a nucleophile and attack an alkyl halide in an S N 2
In this chapter, we have seen that an acetylide ion can function as a nucleophile and attack an alkyl halide in an SN2 process. More generally speaking, the acetylide ion can attack other electrophiles as well. For example, we will see in Chapter 14 that epoxides function as electrophiles and are subject to attack by a nucleophile. Consider the following reaction between an acetylide ion (the nucleophile) and an epoxide (the electrophile):
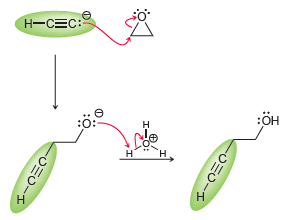
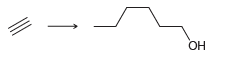
The acetylide ion attacks the epoxide, opening up the strained, three-membered ring and creating an alkoxide ion. After the reaction is complete, a proton source is used to protonate the alkoxide ion. In a synthesis, these two steps must be shown separately, because the acetylide ion will not survive in the presence of H3O+. Using this information, propose a plausible synthesis for the following compound using acetylene as your only source of carbon atoms:
-: :
Step by Step Solution
3.29 Rating (164 Votes )
There are 3 Steps involved in it
H Lindlars Catalys... View full answer

Get step-by-step solutions from verified subject matter experts


