Provide an IUPAC name for each of the following alcohols: a. Br Br
Question:
a.
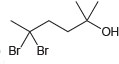
b.
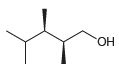
c.
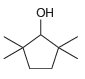
d.
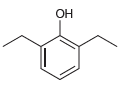
e.
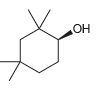
Transcribed Image Text:
"ОН Br Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a 5 5dibromo2methylhexan2ol b ...View the full answer

Answered By

John Aketch
I am a dedicated person with high degree of professionalism, particularly in academic writing. My desire is to is to make students excel in their academic endeavor.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Provide an IUPAC name for each of the following alcohols: a. b. c. d. e. HO. Br Br
-
Provide an IUPAC name for each of the following compounds. a. b. c. d. e. I
-
Give the IUPAC name for each of the following hydrocarbons. a. b. c. d. , , CH3 CH3 CH3CCH2CH2CH2CCH3 CH3 CH3 CH CH2CHCH2CH2CH2CH3 CH2CH2CH3 CH3 CH CHCHCH2CH2CH2 CH3 CH2CH3
-
Aria Perfume, Inc.. sold 3,210 boxes of white musk soap during January of 2016 at the price of $90 per box. The company offers a full refund for any product returned within 30 days from the date of...
-
Prove Markov's Inequality: If X is any random variable and a > 0, then Pr( |X| a) E( |X| )/a. Show how this inequality can be applied to Theorems 5.2 and 5.3.
-
An ongoing discussion among business managers is the return on employee investment (ROEI). Employers want to maximize business profitability, and employees are a significant part of organizational...
-
8-1. Market segmentation involves aggregating prospective buyers into groups that have two key characteristics. What are they?
-
Currently, at a price of $1 each, 100 popsicles are sold per day in the perpetually hot town of Rostin. Consider the elasticity of supply. In the short run, a price increase from $1 to $2 is...
-
Use the information provided below to answer the following questions. INFORMATION As a management accountant, you have recently been recruited to be part of the strategic management accounting team...
-
Jordan Wing, Inc., a sporting goods retailer, began operations on January 2, 2012. It reported net income of $3,091,660 during 2014. Additional information about transactions occurring in 2014...
-
The institution of teacher tenure is meant to a. Ensure job security for teachers with 10 years of experience. b. Ensure that teachers do not get fired for political reasons. c. Allow teachers to...
-
Determine the electron configuration for each of the following ions: a. A carbon atom with a negative charge b. A carbon atom with a positive charge c. A nitrogen atom with a positive charge d. An...
-
In determining cost of goods sold: a. purchases discounts are deducted from net purchases. b. freight-out is added to net purchases. c. purchase returns and allowances are deducted from net...
-
In this problem, we consider mild modifications of the standard MDP setting. (a) (10 points) Sometimes MDPs are formulated with a reward function R(s) that depends only on the current state. Write...
-
All-Walnut, Inc. produces two models of bookcases. The bookcases sell for the amount listed in the table below. Each bookcase requires a certain number of labor hours, machine time, and materials...
-
Problem 1 Find the number of degrees of freedom of the mechanisms (a)-(d) (a) (b)
-
3) (10 pts) The following grammar is given E EAE (E) -E | id V={E,A), T={-,(,),*,/,+,id} and starting symbol is E. a) Give the left-most derivation of w= id+id*id. Is w accepted? b) Is this a...
-
4. X, the proprietor of a departmental store, decided to calculate separate profits for his two departments L and M for the month ending 31st January. Stock on 31st January could not be valued for...
-
Factor each trinomial. k 2 - 11hk + 28h 2
-
Find the numerical value of each expression. (a) sech 0 (b) cosh -1 1
-
Alcohols can act either as weak acid or as weak bases, just as water can. Show the reaction of methanol, CH 3 OH, with a strong acid as HC1 and with a strong base such as NA + - NH 2 .
-
The O ? H hydrogen in acetic acid is much more acidic than any of the C ? H hydrogens. Explain this result using resonance structures. Acetic acid
-
Write the products of the following acid-base reactions: (a) CH3OH + H2SO4 ? (b) CH3OH + NaNH2 ? (c) CH3NH3 + C1- +NAOH ?
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


