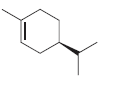
(R)-Limonene is found in many citrus fruits, including oranges and lemons: For each of the following compounds...
Question:
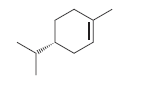
For each of the following compounds identify whether it is (R)-limonene or its enantiomer, (S)-limonene:
a.
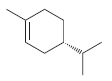
b.
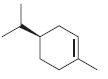
c.
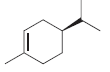
d.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a Slimonene ...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following compounds, identify the expected chemical shift for each type of proton: (a) (b) (c) (d)
-
For each of the following compounds, identify whether each C R C system is cumulated, conjugated, or isolated: (a) (b) (c) (d) HO HO- cis-Aconitic acid Plays a role in the citric acid cycle
-
For each of the following compounds, draw the form in which it will predominate at pH = 3, pH = 6, pH = 10, and pH = 14: a. b. c. CH3COOH pKa = 4.8 CH3CH2NH3 pKa 11.0 CF CH2OH pKa 12.4
-
In Exercises 1 through 14, compute the indicated values of the given function. f(x) = 3x 2 + 5x 2; f(0), f(2), f(1)
-
Submit a paper naming the four major outlaw motorcycle gangs that operate in the United States and discuss the structure of their organizations as well as the similarities between them.
-
White Company has two departments, Cutting and Finishing. The company uses job-order costing and computes a predetermined overhead rate in each department. The Cutting Department bases its rate on...
-
Work out the MHR for the following machine for January 1989. Cost of the machine Rs 90,000 Freight and installation Rs 10,000 Working life 10 years Working hours 2,000 per annum Repair charges 50% of...
-
Gonzalez Corporation needs to set a target price for its newly designed product EverReady. The following data relate to this new product. The costs shown above are based on a budgeted volume of...
-
Atlantic Surf manufactures surfboards. The company's sales budget for the next three months is shown below. In addition, company policy is to maintain finished goods inventory equal (in units) to 40%...
-
A uniformly charged disk like the disk in Fig. 21.26 has radius 2.50 cm and carries a total charge of 4.0 X 10-12 C. (a) Find the electric field (magnitude and direction) on the x-axis at x = 20.0...
-
When 0.075 g of penicillamine is dissolved in 10.0 mL of pyridine and placed in a sample cell 10.0 cm in length, the observed rotation at 20C (using the D line of sodium) is -0.47. Calculate the...
-
Each of the following compounds possesses a plane of symmetry. Find the plane of symmetry in each compound. In some cases, you will need to rotate a single bond to place the molecule into a...
-
Find the acute angle , to the nearest tenth of a degree, for the given function value. sec = 1.279
-
10.) Steam enters a well-insulated turbine at 6 MPa, 400C and expands to 200 kPa, saturated vapor at a rate of 10 kg/s. (a) Draw a schematic of the process (5 pts). (b) Determine the exergy...
-
4. [8 marks] The tides in the Bay of Fundy are some of the largest in the world. The height, h(t), of the tide in meters after t hourse can be modeled by 39 h(t) = 25 con (77) + 30 4 COS 6 (a) What...
-
Wolfe, Inc. had credit sales for the period of $144,000. The balance in Allowance for Doubtful Accounts is a debit of $653. If Wolfe estimates that 2% of credit sales will be uncollectible, what is...
-
Water at 20C is to be pumped from a reservoir (ZA = 5 m) to another reservoir at a higher elevation (ZB = 13 m) through two 36-m- long pipes connected in parallel as shown. The pipes are made of...
-
Delph Company uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 53,000 machine-hours would...
-
Complete each of the following for f(x). (a) If possible, evaluate f(0) and f(-2). (b) Sketch a graph of f. Give the domain and range. (c) Over what interval(s) is the graph of y = f(x) increasing?...
-
Could the owner of a business prepare a statement of financial position on 9 December or 23 June or today?
-
When an aldehyde or a ketone is condensed with ethyl a-chloroacetate in the presence of sodium ethoxide, the product is an α,β-epoxy ester called a glycidic ester. The...
-
The Perkin condensation is an aldol-type condensation in which an aromatic aldehyde (ArCHO) reacts with a carboxylic acid anhydride, (RCH2CO)2O, to give an a,b-unsaturated acid (ArCH "CRCO2H). The...
-
(a) Infrared spectroscopy provides an easy method for deciding whether the product obtained from the addition of a Grignard reagent to an α,β-unsaturated ketone is the...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


