Question: Would you expect the widths of three chi states listed in Table 6.14 to be bigger, smaller or about the same as the widths of
Would you expect the widths of three chi states listed in Table 6.14 to be bigger, smaller or about the same as the widths of the J/ψ(3097) and ψ(3686)? Check your answer with the experimental widths given in the PDG tables.
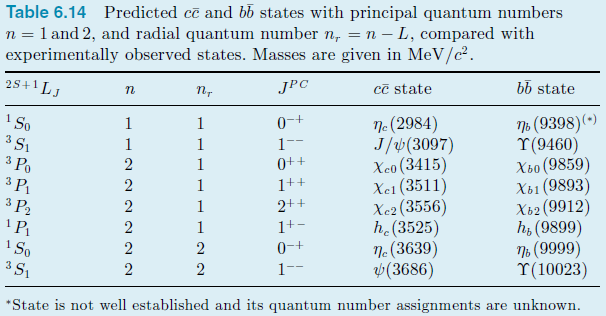
Table 6.14 Predicted cc and bb states with principal quantum numbers n = 1 and 2, and radial quantum number n, = n L, compared with experimentally observed states. Masses are given in MeV/c. 25+1LJ JPC bb state cc state , 1 So 3 S1 3 Po 3 P 3 P2 76 (9398)(-) Y(9460) Xb0 (9859) (9893) 2 (9912) h (9899) (9999) Y(10023) Ne (2984) J/(3097) Xco (3415) Xe1 (3511) Xe2 (3556) h (3525) Ne (3639) (3686) 1-- 2 0++ 1++ 2 2++ 1+- 1 So 3 S, 2 0-+ 2 2 1-- *State is not well established and its quantum number assignments are unknown.
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
The chi states would be expected to have broader widths because they have ... View full answer

Get step-by-step solutions from verified subject matter experts


