At first glance, the structure of diborane would seem unusual. Why shouldnt the molecule assume the same
Question:
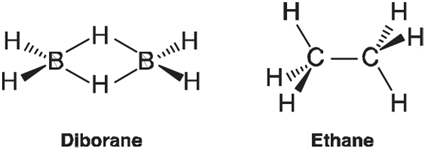
The important difference between the two molecules is that diborane has two fewer electrons than ethane and is not able to make the same number of bonds. In fact, it is ethene which shares the same number of electrons, to which diborane is structurally related.
Obtain equilibrium geometries for both diborane and ethene using the HF/6-31G* model and display the six valence molecular orbitals for each. Associate each valence orbital in ethene with its counterpart in diborane. Focus on similarities in the structure of the orbitals and not on their position in the lists of orbitals. To which orbital in diborane does the π orbital in ethene (the HOMO) best relate? How would you describe this orbital in diborane? Is it B~B bonding, B~H bonding, or both?
Step by Step Answer:






