The mean activity coefficients for aqueous solutions of NaCl at 25C are given below. Confirm that they
Question:
The mean activity coefficients for aqueous solutions of NaCl at 25°C are given below. Confirm that they support the Debye–Hückel limiting law and that an improved fit is obtained with the extended law.
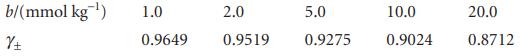
Transcribed Image Text:
b/(mmol kg ¹) 土 1.0 0.9649 2.0 0.9519 5.0 0.9275 10.0 0.9024 20.0 0.8712
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Solution Here we know k A 12ln 12k A 05885 ln 1 04021 ln 2 Since the DebyeHckel law is v...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The mean activity coefficients for aqueous solutions of NaCl at 25 C are given opposite. Confirm that they support the DebyeHckel limiting law and that an improved fit is obtained with the Davies...
-
The volume of an aqueous solution of NaCl at 25 C was measured at a series of molalities b, and it was found that the volume fitted the expression v=1003+16.62x+1.77x 3/2 +0.12x 2 where v=V/cm 3 , V...
-
The mean activity coefficients of KCI in three dilute aqueous solutions at 25C are 0.927 (at 5.0 mmol kg-I), 0.902 (at 10.0 mmol kg-I), and 0.816 (at 50.0 mmol kg"). Estimate the value of B in the...
-
Zolezzi Incorporated is preparing its cash budget for March. The budgeted beginning cash balance is $29,000, Budgeted cash receipts total $100,000 and budgeted cash disbursements total $91,000. The...
-
Why does a dealer offer to trade only a fixed amount at the bid and ask prices?
-
Explain what is meant by repudiation and, in particular, the effect of anticipatory breach on the position of the parties to a contract.
-
Describe any way in which you have used information technology to make you more productive.
-
1. Which of the following is a responsibility of a local office of the IRS? a. Advising the Treasury Department on legislation b. Intelligence operations c. Appellate procedures d. Developing IRS...
-
Hello, I upvote if answers are correct and organized. Characteristics of Production Process, Cost Measurement Vince Melders, of EcoScape Company, designs and installs custom lawn and garden...
-
The Two Variable Regression for the regression model y = + x + (a) Show that the least squares normal equations imply iei = 0 and i xi ei = 0. (b) Show that the solution for the constant term is a...
-
The enthalpy of fusion of anthracene is 28.8 kJ mol 1 and its melting point is 217C. Calculate its ideal solubility in benzene at 25C.
-
The osmotic pressure of solutions of polystyrene in toluene were measured at 25C and the pressure was expressed in terms of the height of the solvent of density 1.004 g cm 3 : Calculate the molar...
-
What is meant by the cyclically-adjusted primary budget deficit? How can it be calculated? Why is it conceptually equivalent to the discretionary fiscal impulse? Is such a deficit sustainable?
-
A. Use the following information to answer the six questions below. Variable Manufacturing Cost Per Unit20 Variable selling cost per unit25 Selling Price per unit100 Fixed Manufacturing cost per unit...
-
The team has been charged with reviewing quarterly results for the LusterLast moisturizing shampoo, called SatinSmooth. The product is new to the line and is sold mostly in drugstores and grocery...
-
Problem 4 (25 pts.) Consider the function f(x, y) = xy y +2. (i) (5 pts) Find the gradient of f (ii) (10 pts) Find the directional derivative of f at the point (1,2) in the direction of the vector...
-
PROBLEM 4. (15 points) a) Determine the range of charged particles emitted from Phosphorus-32 in iron. (5 points) b) Determine the necessary thickness of an iron plate to attenuate the flux of...
-
(b) In the case of no losses, Moody (1965) recommends the following equation for calculating the mass flow rate of wet steam (ie. two-phase water) through the constriction =A 2(h-h) Variable and...
-
a. Determine an equation of the tangent line and normal line at the given point (x 0 , y 0 ) on the following curves. b. Graph the tangent and normal lines on the given graph. x 4 = 2x 2 + 2y 2 ; (x...
-
A circular concrete shaft liner with Youngs modulus of 3.4 million psi, Poissons ratio of 0.25, unconfined compressive strength 3,500 psi and tensile strength 350 psi is loaded to the verge of...
-
As a variation of the preceding problem explore the consequences of increasing the energy separation of the B and C orbitals (use S=0 for this stage of the calculation). Are you justified in ignoring...
-
Write down the secular determinants for (i) anthracene (1), (ii) phenanthrene (2) within the Hckel approximation and using the C2p orbitals as the basis set. 1 Anthracene 2 Phenanthrene
-
What is the physical significance of the Coulomb and resonance integrals?
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...

Study smarter with the SolutionInn App


