The osmotic pressure of solutions of polystyrene in toluene were measured at 25C and the pressure was
Question:
The osmotic pressure of solutions of polystyrene in toluene were measured at 25°C and the pressure was expressed in terms of the height of the solvent of density 1.004 g cm−3:
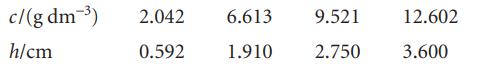
Calculate the molar mass of the polymer.
Transcribed Image Text:
c/(g dm-³) h/cm 2.042 6.613 0.592 1.910 9.521 2.750 12.602 3.600
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
Assuming molar mass x then since CRT wh...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 298 K, the osmotic pressure of a glucose solution is 10.50 atm. Calculate the freezing point of the solution. The density of the solution is 1.16 g/mL.
-
The concentration dependence of the osmotic pressure of solutions of a macromolecule at 20C was found to be as follows: Determine the molar mass of the macromolecule and the osmotic virial...
-
The osmotic pressure of 0.010 M solutions of CaCl2 and urea at 25C are 0.605 atm and 0.245 atm, respectively. Calculate the van't Hoff factor for the CaCl2 solution.
-
A geostationary satellite orbiting at a height 36,000km operates at 4GHz using a transmitter antenna that has a gain of 15dB. On earth the receiver antenna has a gain of 45dB. Calculate; (a) Free...
-
The SEC regulates American stock markets. However, NYSE members have committees that carry out a host of self-regulatory activities. NYSE members are profit seekingwhy would they self-regulate...
-
Discuss the reasons why expatriate assignments fail and what is being done to reduce the failure rate.
-
Interview a person in a high-pressure job in any field. Find out whether the person experiences significant stress and what method he or she uses to cope with it.
-
(a) Belinda believes that the couple could maintain their current level of living if their retirement income represented 75 percent of their current annual income after adjusting for inflation....
-
1.Is the accounting for revenue in the Victor transaction a case of operational earnings management or accounting earnings management? Explain. Briefly address auditing challenges of this kind of...
-
The following December 31, 2021, fiscal year-end account balance information is available for the Stonebridge Corporation: Cash and cash equivalents ......................................$ 5,000...
-
The mean activity coefficients for aqueous solutions of NaCl at 25C are given below. Confirm that they support the DebyeHckel limiting law and that an improved fit is obtained with the extended law....
-
A water carbonating plant is available for use in the home and operates by providing carbon dioxide at 5.0 atm. Estimate the molar concentration of the soda water it produces.
-
Explain why, in a fixed-rate mortgage, the amount of the mortgage payment applied to interest declines over time, while the amount applied to the repayment of principal increases.
-
PAYMENTS DURING 2022/23 DATE DESCRIPTION FULL YEAR Private Hospital Insurance Premiums FULL YEAR Childcare costs FULL YEAR FULL YEAR FULL YEAR 18/08/22 27/08/22 01/09/22 01/10/22 01/11/22 01/12/22...
-
been called Recently, s asked to hington, ent of the in areas: g costs, and pany's a simple 12. The Tru-Green Lawn Company provides yard care services for customers throughout the Denver area. The...
-
During the week of November 12, 2021, Ernestina Manufacturing produced abd shipped 7,500 units of its aluminum wheels: 1,500 units of Model A and 6,000 units of Model B. The following costs were...
-
Daicos Ltd is a public company that competes in the highly competitive market for manufactured household products. The company is dominated by Peter Daicos, the chairman and chief executive officer,...
-
Hypothesis testing A tire company claims that a new range on average lasts at least 28,000 km. Tests with 64 tires result in an average duration of 27,800 km. With a standard deviation of 1,000 km....
-
a. Determine an equation of the tangent line and normal line at the given point (x 0 , y 0 ) on the following curves. b. Graph the tangent and normal lines on the given graph. 3x 3 + 7y 3 = 10y; (x 0...
-
Cornell and Roberts are partners who agree to admit Stanley to their partnership. Cornell has a capital balance of $80,000 and Roberts has a capital balance of $120,000. Cornell and Roberts share net...
-
In a particular photoelectron spectrum using 21.21 eV photons, electrons were ejected with kinetic energies of 11.01 eV, 8.23 eV, and 5.22 eV. Sketch the molecular orbital energy level diagram for...
-
Evaluate the bond order of each Period 2 homonuclear diatomic molecule.
-
Set up the secular determinants for the homologous series consisting of ethene, butadiene, hexatriene, and octatetraene and diagonalize them by using mathematical software. Use your results to show...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...

Study smarter with the SolutionInn App


