The squares of a number of vibrational energy eigenfunctions are shown superimposed on a Morse potential in
Question:
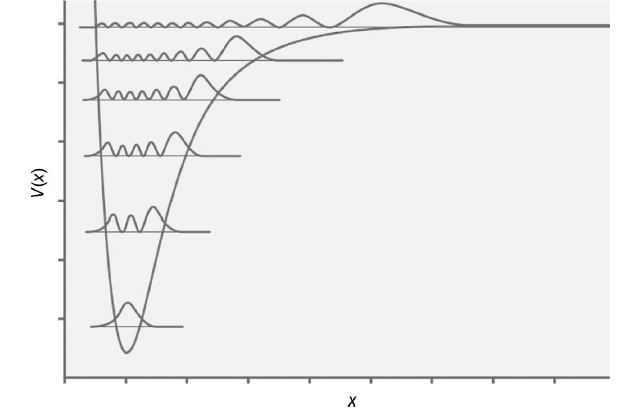
Transcribed Image Text:
franna (x)A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The quantum number is the number of nodes or 0 2 4 6 8 and 11 from the l...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Overtone transitions in vibrational absorption spectra for which În = +2, + 3, ¦ are forbidden for the harmonic potential V = (1 2)kx 2 because μ mn x = 0 for £m...
-
The total energy eigenvalues for the hydrogen atom are given by E n = e 2 / (8Ïε 0 a 0 n), n = 1, 2, 3, 4,¦, and the three quantum numbers associated with the total energy...
-
In this problem, you will solve for the total energy eigenfunctions and eigenvalues for an electron in a finite depth box. We first go through the calculation for the box parameters used in Figure...
-
A specific purpose statement is not stated in the speech but includes both the general purpose and the topic. It often reveals the intended goal for the audience. O True False
-
In Exercises 1-4, solve the equation. 1. sin 2x sin x = 0 2. sin 2x sin x = cos x 3. cos 2x cos x = 0 4. cos 2x + sin x = 0
-
Using the method of Section 4.7, solve Problem 4.22. PROBLEM 4.22 A lever AB is hinged at C and attached to a control cable at A. If the lever is subjected to a 500-N horizontal force at B, determine...
-
What are we going to make? LO.1
-
The administrator of elections for the city of Crossville has been asked to perform an activity analysis of its optical scanning center. The optical scanning center reads voter forms into the...
-
On January 1, 2017, Learned Inc, issued $16 million face amount of 20-year, 18% stated rate bonds when market interest rates were 20%. The bonds pay interest semiannually each June 30 and December 31...
-
Write pseudocode agent programs for the goal-based and utility-based agents. The following exercises all concern the implementation of environments and agents for the vacuum-cleaner world.
-
The number of molecules in a given energy level is proportional to e E kBT , where E is the difference in energy between the level in question and the ground state. How is it possible that a...
-
As a diatomic molecule rotates, the centrifugal force leads to a small change in the bond length. Do you expect the bond length to increase or decrease? Do you expect the difference between adjacent...
-
We defined the relaxation of the 8-puzzle in which a tile can move from square A to square B if B is blank. The exact solution of this problem defines Gaschnigs heuristic (Gaschnig, 1979). Explain...
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
An amount of money is invested at 4% annual simple interest, and twice that amount is invested at 5%. The total annual interest is $112. How much is invested at each rate?
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
A 1.50-L mixture of helium, neon, and argon has a total pressure of 754 mmHg at 310 K. If the partial pressure of helium is 431 mmHg and partial pressure of neon is 211 mmHg, what mass of argon is...
-
Methanol (CH 3 OH) can be synthesized by the reaction: CO(g) + 2 H 2 (g) CH 3 OH(g) What volume (in liters) of methanol gas, measured at a temperature of 473 K and a pressure of 820 mmHg, is...
-
How many grams of water form when 2.41 L of oxygen gas at STP completely react with an appropriate amount of H 2 ? 2 H 2 (g) + O 2 (g) 2 H2O(g)
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


