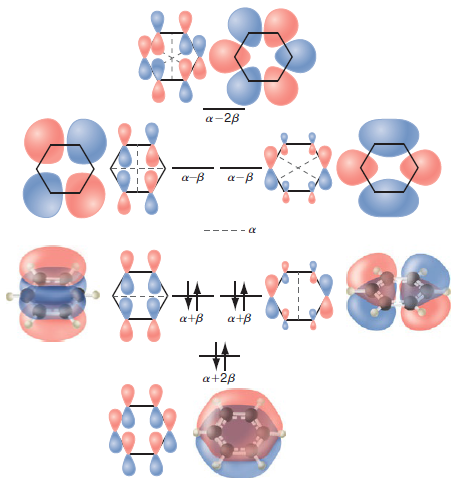
What is the in-plane amplitude of the wave functions describing the Ï network in the conjugated molecules
Question:
Transcribed Image Text:
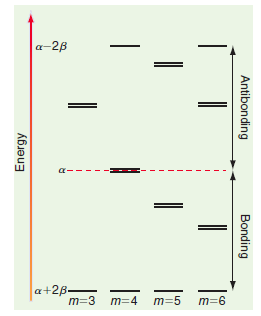
a-2B a-B a+B a+B a+2B a-2B a+2B- m=3 m=4 m=5 m=6 Antibonding Bonding || Energy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 30% (10 reviews)
The Huckel model is valid for a planar conjugated molec...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In this problem, you will solve for the total energy eigenfunctions and eigenvalues for an electron in a finite depth box. We first go through the calculation for the box parameters used in Figure...
-
The amplitude of the wave on the right side of the barrier in Figure 16.10 is much smaller than that of the wave incident on the barrier. What happened to the rest of the wave? Figure 16.10 Vo E= 1/4...
-
The wave function of a standing wave is y(x, t) = 4.44 mm sin [(32.5 rad/m) x] sin [(754rad/s) t]. For the two traveling waves that make up this standing wave, find the (a) Amplitude; (b) Wavelength;...
-
An interior room is maintained at an air temperature of 210C by a radiant panel covering one of the room walls, calculate the temperature of the radiant panel necessary to achieve the thermal comfort...
-
describe the relationship between the graphs of f and g. Consider amplitude, period, and shifts. 1. f (x) = cos x g(x) = cos 5x 2. f (x) = sin x g(x) = 2 sin x 3. f (x) = cos 2x g(x) = cos 2x 4. f...
-
Suppose = /2 is the only solution of a trigonometric equation in the interval 0 < 2. Assuming a period of 2, which of the following formulas gives all solutions of the equation, where k is an...
-
Let dMi = i dBi for i = 1,2 and Brownian motions B1 and B2. Suppose 1 and 2 satisfy condition (12.5), so M1 and M2 are finite-variance martingales. Consider discrete dates s = t0 < t1 < < tN = u for...
-
Assume that a 1.00-kg ball is thrown solely by the action of the forearm, which rotates about the elbow joint under the action of the triceps muscle, Fig. 8-45. The ball is accelerated uniformly from...
-
The St. Lucia Blood Bank, a private charity partly supported by government grants, is located on the Caribbean island of St. Lucia. The blood bank has just finished its operations for September,...
-
Reconsider the system defect situation described in Exercise 26 (Section 2.2). In Exercise 26 A certain system can experience three different types of defects. Let Ai (i = 1,2,3) denote the event...
-
The angular functions, Π(θ)Φ(), for the one-electron HartreeFock orbitals are the same as for the hydrogen atom, and the radial functions and radial probability...
-
What is the rational for setting H ij = 0 for nonadjacent atoms in the Huckel model?
-
A construction worker uses a bosun's chair arrangement (as shown in the figure (Figure 1)) to lift himself to his workplace. The rope may be considered massless and the pulley may be considered...
-
You will be creating a Performance Improvement Plan to address an employee in the attached case study (see below). This is a scenario you may encounter in your future HR profession, so this...
-
For this prompt, consider your academic goals, including (but not limited to) such topics as how you plan to manage your time to fit in your studies; how you will build your skills, as needed; how...
-
1. An introduction of you as a leader (whether or not you see yourself as a leader, whether or not you like being a leader, what kinds of leadership roles you have had, etc.). 2. Summarize your...
-
Briefly, describe the firm in terms of the following items. a. Size in terms of market capitalization, annual revenue, number of employees, location(s). b. Discuss the financial position of the firm....
-
HealthyLife (HL) is a publicly-traded company in the Food Manufacturing Industry. HealthyLife has been around since the 1970s, and is mainly focused on the production and wholesale of "organic and...
-
In Exercises 1 through 18, differentiate the given function. y 3 x + 5
-
Players A, B, and C toss a fair coin in order. The first to throw a head wins. What are their respective chances of winning?
-
Calculate the electronic contribution to the molar internal energy at 1900 K for a sample composed of the atoms specified in Exercise 16.4a. Data in Exercise 16.4a. A certain atom has a threefold...
-
By what factor does the number of available configurations increase when 20 m 3 of air at 1.00 atm and 300 K is allowed to expand by 0.0010 per cent at constant temperature?
-
A certain atom has a threefold degenerate ground level, a nondegenerate electronically excited level at 3500 cm 1 , and a threefold degenerate level at 4700 cm 1 . Calculate the partition function of...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


