Draw a series of pictures, similar to Figure 41.21, for the ground states of Ca, Ni, As,
Question:
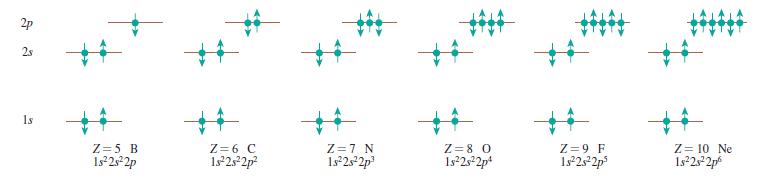
Draw a series of pictures, similar to Figure 41.21, for the ground states of Ca, Ni, As, and Kr.
Figure 41.21:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
For the ground state ...View the full answer

Answered By

Sheeba Khan
I have been a chemistry teacher since 2014 and started my career as an online tutor in 2018. I love to study and teach chemistry. It is my passion.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
Draw a series of pictures, similar to Figure 41.22, for the ground states of Ca, Ni, As, and Kr. 2p 2s %23 %23 1s Z = 5 B 1s25 2p Z = 6 C 1s 2s2p? Z =7 N 1s 2s2p Z = 8 0 1s2s2p* Z = 9 F 1s 2s2p Z =...
-
Draw a series of pictures, similar to Figure 41.22, for the ground states of K, Sc, Co, and Ge. 2p 2s %23 %23 1s Z = 5 B 1s25 2p Z = 6 C 1s 2s2p? Z =7 N 1s 2s2p Z = 8 0 1s2s2p* Z = 9 F 1s 2s2p Z = 10...
-
Draw a series of pictures, similar to Figure 41.21, for the ground states of K, Sc, Co, and Ge. Figure 41.21:
-
If you can't find similar ratios to class-covered ones, use financial formulas to calculate them manually. Choose up to two of the following: Operating Margin EBITDA Margin Payout Ratio 3. Analysis...
-
A variable of two populations has a mean of 40 and a standard deviation of 12 for one of the populations and a mean of 40 and a standard deviation of 6 for the other population. a. For independent...
-
How could Koalas managers improve the management of overhead costs?
-
Prepare projected financial statements for a business and interpret their significance for decision-making purposes.
-
The following were among the transactions of Kenton Company during this year. The firm, whose fiscal year ends on December 31, uses a periodic inventory system. May 24 Gave a 60-day, 5.5 percent...
-
On January 1, 2019, Everlasting, Inc. purchased Comet Corporation for $650,000. On that date the net assets of Comet had a book value of $320,000, and book values were equal to fair values with the...
-
NoNuns Cos. has a 25 percent tax rate and has $350 million in assets, currently financed entirely with equity. Equity is worth $37 per share, and book value of equity is equal to market value of...
-
Figure 41.22 shows that the ionization energy of cadmium (Z = 48) is larger than that of its neighbors. Why is this? Figure 41.22:
-
a. Draw a diagram similar to Figure 41.2 to show all the possible orientations of the angular momentum vector L for the case l = 3. Label each L with the appropriate value of m. b. What is the...
-
This chapter explained the purpose of managerial accounting in the context of the current business environment. Review the automobile section of your local newspaper; the Sunday paper is often best....
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Estimate the hydrogen consumption required to completely remove the sulfur from a hydrotreater feedstock and to reduce the nitrogen content of the product to 15 ppm by weight. The 48.5 API naphtha...
-
2. Consider the following kinds of information, and suggest the most appropriate data type to store or represent each: Information Suggested Data Type String A person's name A person's age in years A...
-
Steam at 32 MPa, 520C enters the first stage of a supercritical reheat cycle including three turbine stages. Steam exiting the first-stage turbine at pressure p is reheated at constant pressure to...
-
Why do you think that Java provides no constructors for the InetAddress class?
-
Decades after the event, Johnson & Johnson (J&J), the 130-year-old American multinational, is still praised for swiftly The company indicated that its response was based on the expectations set forth...
-
Which of the following transitions in sodium do not occur as electric dipole transitions? (Give the selection rule that is violated.) 451/2 3S1/2 4D3/23P1/2 4S1/2 3P3/2 4D3/2 351/2 4P3/2 3S1/2 5D3/2...
-
Write the ground-state electron configuration of (a) Carbon, (b) Oxygen, and (c) Argon.
-
Five identical noninteracting particles are place in an infinite square well with L = 1.0 nm. Compare the lowest total energy for the system if the particles are (a) Electrons and (b) Pions. Pions...
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


