Question: FIGURE Q40.7 shows two possible wave functions for an electron in a linear triatomic molecule. Which of these is a bonding orbital and which is
FIGURE Q40.7 shows two possible wave functions for an electron in a linear triatomic molecule. Which of these is a bonding orbital and which is an antibonding orbital? Explain how you can distinguish them.
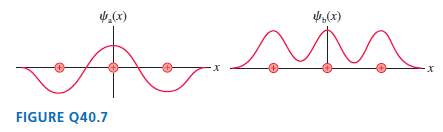
.(x) ,(x) FIGURE Q40.7
Step by Step Solution
3.44 Rating (176 Votes )
There are 3 Steps involved in it
Wave function a is an antibonding orbital and wave ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778c02f76_701153.pdf
180 KBs PDF File
1442_6054778c02f76_701153.docx
120 KBs Word File


