Question: An eccentrically loaded foundation is shown in Figure P6.11. Use FS of 4 and determine the maximum allowable load that the foundation can carry. Use
An eccentrically loaded foundation is shown in Figure P6.11. Use FS of 4 and determine the maximum allowable load that the foundation can carry. Use Meyerhof’s effective area method.
Figure P6.11.
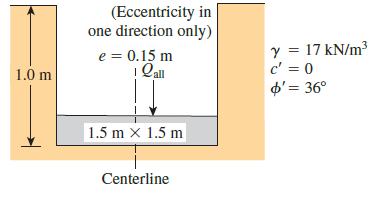
(Eccentricity in one direction only) Y = 17 kN/m c' = 0 o'= 36 e = 0.15 m %3D 1.0 m 1.5 m x 1.5 m Centerline
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Given data Meyerhofs effective area method FS 4 1 Determine A B and L The foundation is with oneway ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
2049_61d6ac342b8fc_836482.pdf
180 KBs PDF File
2049_61d6ac342b8fc_836482.docx
120 KBs Word File


