A gas chromatogram of a mixture of toluene and ethyl acetate is shown here. (a) Use the
Question:
A gas chromatogram of a mixture of toluene and ethyl acetate is shown here.
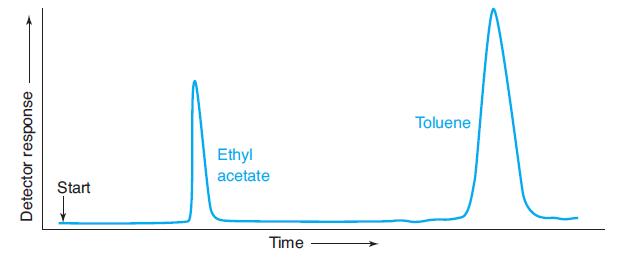
(a) Use the width of each peak (measured at the base) to calculate the number of theoretical plates in the column. Estimate all lengths to the nearest 0.1 mm.
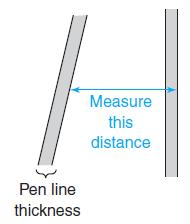
(b) Using the width of the toluene peak at its base, calculate the width expected at half-height. Compare the measured and calculated values. When the thickness of the line is significant relative to the length being measured, it is important to take the pen line width into account. You can measure from the edge of one line to the corresponding edge of the other line, as shown here.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





