Why are La and Lb peaks, but not Ka and Kb peaks for lead identified in Figure
Question:
Why are La and Lb peaks, but not Ka and Kb peaks for lead identified in Figure 21-31? Why are La and Lb peaks not identified for iron in Figure 21-31?
In Figure 21-31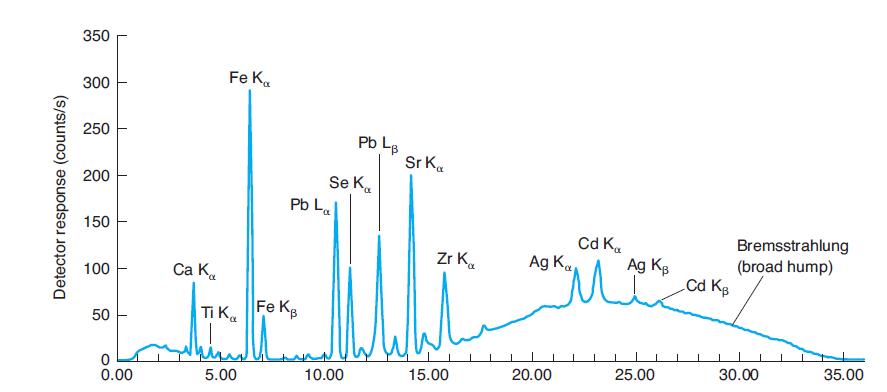
Transcribed Image Text:
350 Fe K. 300 250 Pb L. Sr K. Se K. Pb La 200 150 Cd K. Ag K Bremsstrahlung (broad hump) Cd Kg Zr K. Ag Kg 100 Ca K. Ti Ka Fe KB 50 25.00 30.00 35.00 5.00 10.00 15.00 20.00 0.00 Detector response (counts/s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
Answer There are four fundamental processes that will p...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Why will a block of iron float in mercury but sink in water?
-
Why do maximizing EPS and maximizing value not necessarily lead to the same conclusion about the optimal capital structure?
-
Why are the multiplet splittings in Figure 28.9 not dependent on the static magnetic field? J12 J42 (yB/2m)(01- 2) Frequency Intensity -----
-
Wollongong Group Ltd, of New South Wales, Australia, acquired its factory building about 10 years ago. For several years the company has rented out a small annex attached to the rear of the building....
-
If you were Erin Dempsey, what would you do?
-
A log weighing 800 lb is lifted by a pair of tongs as shown. Determine the forces exerted at E and at F on tong DEF.
-
In Exercise 7.29 (p. 371), the inflation forecasts of nine economists that were made in June 1999 and January 2000 were reported. These forecasts, obtained from the Wall Street Journal, are...
-
The costs of rework are always charged to the specific jobs in which the defects were originally discovered. Do you agree? Explain.
-
Miles Cyprus Corp. purchased a truck that currently has a book value of $1,000. If the firm sells the truck for $5,000 today then what is the amount of cash that it will net after taxes if the firm...
-
Dry Quick (DQ) is a medium-sized, private manufacturing company located near Timmins, Ontario. DQ has a June 30 year-end. Your firm, Poivre & Sel (P&S), has recently been appointed as auditors forDQ....
-
Where would Kb emission peaks for Ti, Se, and Zr be found in Figure 21-31? Why are they not labeled? In Figure 21-31 350 Fe K. 300 250 Pb L. Sr K. Se K. Pb La 200 150 Cd K. Ag K Bremsstrahlung (broad...
-
How much energy in kJ/mol is released when nitrogen emits Ka radiation at 0.392 keV? Compare the Ka energy to 945 kJ/mol, which is the energy required to break the triple bond in N 2 (one of the...
-
The mileage (in 1,000s of miles) that car owners get with a certain kind of radial tire is a random variable Y having a lognormal distribution such that Y = eX, where X is normally distributed. Let...
-
How do I record these entries? January 1: Purchased a fleet of vehicles for $350,000 via a loan from the bank. The trucks have a useful life of six years. The loan is for six years with an interest...
-
How do feedback mechanisms and performance evaluation systems contribute to individual and team development within a corporate context ?
-
How do ideological frameworks underpin political movements, and what is their role in legitimizing or challenging power structures?
-
According to the 8-step communication model, what should one do once the crisis has passed?
-
Who is PGR and what do they do? How much premium did the company (consolidated) write in the most recent complete fiscal year? Was it a good, average, or bad year for them? Comment on the premium...
-
Reggie, who is 55, had AGI of $32,000 in 2019. During the year, he paid the following medical expenses: Drugs (prescribed by physicians) $500 Marijuana (prescribed by physicians) 1,400 Health...
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
A dibasic compound, B, has pKb1 = 4.00 and pKb2 = 6.00. Find the fraction in the form BH2 2+ at pH 7.00, using Equation 9 - 19. Note that K1 and K2 in Equation 9-19 are acid dissociation constants...
-
Write the chemical reactions whose equilibrium constants are Kb1 and Kb2 for the amino acid proline. Find the values of Kb1 and Kb2.
-
What fraction of ethane-1,2-dithiol is in each form (H2A, HA-, A2-) at pH 8.00? at pH 10.00?
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


