Where would Kb emission peaks for Ti, Se, and Zr be found in Figure 21-31? Why are
Question:
Where would Kb emission peaks for Ti, Se, and Zr be found in Figure 21-31? Why are they not labeled?
In Figure 21-31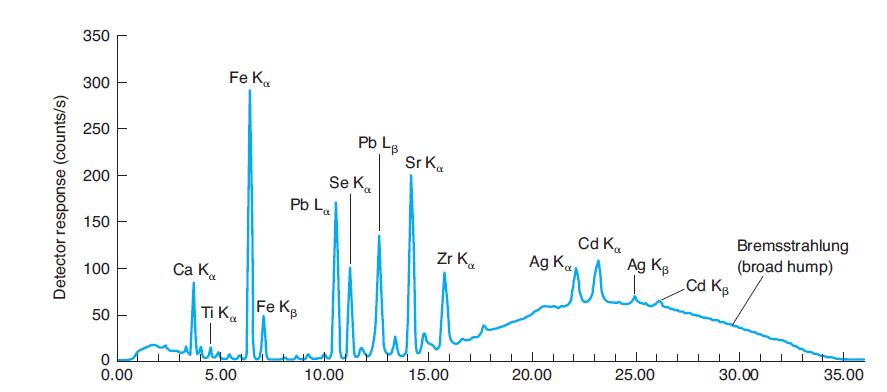
Transcribed Image Text:
350 Fe K. 300 250 Pb L. Sr K. Se K. Pb La 200 150 Cd K. Ag K Bremsstrahlung (broad hump) Cd Kg Zr K. Ag Kg 100 Ca K. Ti Ka Fe KB 50 25.00 30.00 35.00 5.00 10.00 15.00 20.00 0.00 Detector response (counts/s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
For Ti Kbeta line is at 493181kev so next to the ka...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is a hedge fund? Why are they not a recommended investment for most investors?
-
Why are higher resolution monochromators found in ICP atomic emission spectrometers than in flame atomic absorption spectrometers?
-
Why are La and Lb peaks, but not Ka and Kb peaks for lead identified in Figure 21-31? Why are La and Lb peaks not identified for iron in Figure 21-31? In Figure 21-31 350 Fe K. 300 250 Pb L. Sr K. Se...
-
Medallion and RIEF (Renaissance Institutional Equity Fund) are both managed by Renaissance Technologies. How do they differ in terms of asset classes, dollar capacity, average holding period of each...
-
What is an organization's responsibility in regard to sexual harassment among coworkers or supervisor subordinate pairs? Do you think that Daryl Kolendich responded appropriately to the problem?
-
A Mansard roof truss is loaded as shown. Determine the force in members DF, DG, and EG. 12 EN 12 kN 12 KN 1.2 kN 12 kN 4m- 2.25 m 225 m
-
A job-scheduling innovation that has helped managers overcome motivation and absenteeism problems associated with a fixed 8-hour workday is a concept called flextime. This flexible working hours...
-
If researchers know that consumers in various geographic regions respond quite differently to a product category, such as tomato sauce, is area sampling appropriate? Why or why not?
-
Bell Company produces stainless steel drink tumblers, its only product. These tumblers are stamped with company logos and used as promotional items. Following are simplified job cost sheets for two...
-
Explicit expressions for hydrogenic orbitals are given in Tables 10.1 and 9.3. (a) Verify both that the 3px orbital is normalized (to I) and that 3px and 3dxy are mutually orthogonal. (b) Determine...
-
Explain why X-ray fluorescence is observed when matter absorbs X-rays of sufficient energy. Why does each element have a unique X-ray signature?
-
How much energy in kJ/mol is released when nitrogen emits Ka radiation at 0.392 keV? Compare the Ka energy to 945 kJ/mol, which is the energy required to break the triple bond in N 2 (one of the...
-
Nitrogen dioxide decomposes to nitrogen monoxide and oxygen in a second-order reaction. The decrease in concentration of NO 2 is shown as a function of time in the accompanying figure. Use the data...
-
Which topics do you see as being most relevant to your current job or the job you will seek to obtain once you have earned your degree? How so ? In which ways has this course Commercial Law changed...
-
Directions Answer the following reflective questions: There do exist examples of business organizations following principles of behavior that are not entirely self-serving, but rather, are pursuing...
-
10 Count scallops cost $12.97 per pound. How much do they cost for each? A Wagyu Beef New York Strip costs $14 per pound and weighs 15 pounds. The useable yield is 12.5 pounds. How many 12 ounce...
-
How do coordinating agencies differ in a crisis, disaster, and an emergency ?Explain
-
How do we manage and respond to customer feedback and reviews to maintain a positive brand reputation? Explain with the help of examples.
-
Dante and Rosa, both under 65 and married, have a combined AGI of $45,000 in year 2019. Due to certain heart issues, Dante has been prescribed Lipitor by a physician. For year 2019, Dante spent a...
-
What is the difference between the straight-line method of depreciation and the written down value method? Which method is more appropriate for reporting earnings?
-
The diprotic acid H2A has pK1 = 4.00 and pK2 = 8.00. (a) At what pH is [H2A] = [HA-]? (b) At what pH is [HA-] = [A2-]? (c) Which is the principal species at pH 2.00: H2A, HA-, or A2-? (d) Which is...
-
The base B has pKb = 5.00. (a) What is the value of pKa for the acid BH+? (b) At what pH is [BH+] = [B]? (c) Which is the principal species at pH 7.00: B or BH+? (d) What is the quotient [B]/[BH+] at...
-
The acid HA has pKa = 4.00. Use Equations 9-17 and 9-18 to find the fraction in the form HA and the fraction in the form A- at pH = 5.00. Does your answer agree with what you expect for the quotient...
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
Which of the following statement regarding a post-closing trial balance is not true
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions

Study smarter with the SolutionInn App


