Calculate the theoretical masses of the sucralose species in Figure 22-14 at nominal masses of 395,395, 397,397,
Question:
Calculate the theoretical masses of the sucralose species in Figure 22-14 at nominal masses of 395,395, 397,397, 399,399 and 401.401. Find the difference in ppm between observed and calculated m/z.m/z.
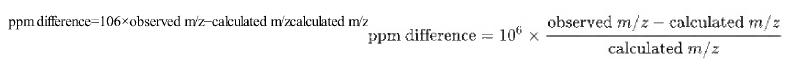
Figure 22-14

Transcribed Image Text:
ppm difference=106xobserved m/z-calculated m/zcalculated m/z ppm difference = 106 observed m/z - calculated m/z calculated m/z
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
Answer Theoretical masses of the sucralose species in Figure 2214 at nominal masses of 39539...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
Calculate the theoretical masses of the species in Figure 21-9 and compare your answers with the values observed in the figure. Figure 21-9 2 000 31p+ 14N16OH* 1 000 15N160* 30.97 30.98 30.99 31.00...
-
In Figure 22-14, the sucralose species with a nominal mass X=395 X=395 is [12C121H1816O835C13]-. [ 12 C 12 1 H 18 16 O 8 35 Cl 3 ]-. X+1X + 1 arises from isotopologues containing one 13C, 13 C, one...
-
The point masses m and 2m lie along the x-axis, with m at the origin and 2m at x = L. A third point mass M is moved along the x-axis. (a) At what point is the net gravitational force on M due to the...
-
How to respond to the following? Cycle stock, pipeline inventory, buffer stock, and in-transit inventory all have associated carrying costs. Interest rates on inventory values, warehousing expenses,...
-
Let the statistic U2 be as defined by Eq. (11.3.32), and let be fixed positive constant. Show that for all observed values (xi , yi), for i = 1, . . . , n, the set of points (0, 1) such that U2 < ...
-
If an oxidation occurs in a reaction, it must be accompanied by a reduction. Is the statement true? Explain why or why not.
-
(e) Is there a significant main effect of Sex or of Diet, or a significant Sex Diet interaction? What interpretation should be placed on significant effects among these terms?
-
Shelf mix decision Superstore is a large discount supermarket. Profits have declined, so the manager has collected data on revenues and costs for different food categories. The following data pertain...
-
Answer: B: Small Product Total Cost After Procurement Total Cost After Stocking Total Cost After Picking Total Cost After Shipping Average Unit Cost Large Product Total Cost After Procurement Total...
-
Use simple decision trees and a 10% per year discount rate to evaluate Project Sable, which has two phases. You may invest in the first, in both or in neither. You may not invest in the second phase...
-
Mass spectral interpretation. The compound C9H4N2Cl6 C 9 H 4 N 2 Cl 6 is a by-product found in chlorinated pesticides. a. Verify that the formula for rings + double bonds agrees with the structure....
-
When looking at the mass spectrum of an unknown substance, you tentatively identify the molecular ion MM as the peak with the most significant intensity at the high-mass end of the spectrum. Then you...
-
For each of the transactions in M3- 10, write the journal entry using the format shown in the chapter. Activity Amount or Explanation a. Swing Hardpaid $450 to its golf instructors for b. Swing Hard...
-
Determine dy/dr when 3x+4y = 3.
-
Problem 3. Doping a Semiconductor The following chemical scheme is used to introduce P-atoms as a dopant into a semiconductor - a silicon chip. POCI3 Cl POCI 3 vapor P P SiO2 + P(s) CVD coating Si...
-
The system shown in the following figure is in static equilibrium and the angle is equal to 34 degrees. Given that the mass1 is 8 kg and the coefficient of static friction between mass1 and the...
-
Pre-Writing step for a report for your boss on Richard Hackman's statement that using a team to complete a complex project may not be the best approach. Review your classmates' contributions to the...
-
For the graph of the equation x = y - 9, answer the following questions: the x- intercepts are x = Note: If there is more than one answer enter them separated by commas. the y-intercepts are y= Note:...
-
A person has severe hearing loss that reduces the intensity in his inner ear by 40 dB. To compensate, a hearing aid can amplify the sound pressure amplitude by a certain factor to bring the intensity...
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
Detection limit. A sensitive chromatographic method was developed to measure sub-part per-billion levels of the disinfectant by-products iodate (IO 3 ), chlorite (ClO 2 ), and bromate (BrO 3 ) in...
-
Olympic athletes are tested to see if they are using illegal performance-enhancing drugs. Suppose that urine samples are taken and analyzed and the rate of false positive results is 1%. Suppose also...
-
Olympic athletes are tested to see if they are using illegal performance-enhancing drugs. Suppose that urine samples are taken and analyzed and the rate of false positive results is 1%. Suppose also...
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....
-
Alado fis istirmerfs Tat likifond 205L [ridont inip lanod whadtinion? hingend is antan Qultit foer avdeed Divdasit errem yodichiders Etexlpoges Getmare nelp
-
The limitation on the deduction of business interest does not apply to non-corporate taxpayers. course hero True or False explain?

Study smarter with the SolutionInn App


