Which is a stronger acid, (a) or (b)? (a) (b) Cl,HCCOH , Dichloroacetic acid Chloroacetic acid K
Question:
Which is a stronger acid, (a) or (b)?
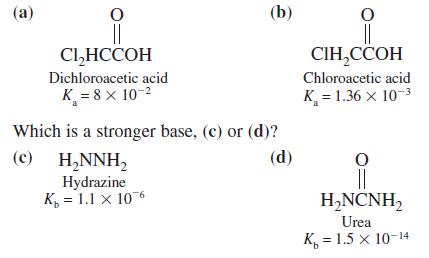
Transcribed Image Text:
(a) (b) Cl,HCCOH СІН,ССОН Dichloroacetic acid Chloroacetic acid K = 8 x 10-2 K = 1.36 x 10-3 Which is a stronger base, (c) or (d)? (c) (d) H,NNH, Hydrazine K, = 1.1 x 106 H,NCNH, Urea K, = 1.5 x 10-14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (18 reviews)
Since Ka is acid dissociation constant so hi...View the full answer

Answered By

PUSHPENDRA JADOUN
I always wanted to be a tutor.
I have been teaching since I was a student. I have experience as a professional tutor of 7 years.
I my experience I have come to understand that by proper mentoring and timing one can achieve his/her goals.
As I have done counseling of a large numbers of students/ parents ,I can say it for sure that education is key to good life
Tutoring have given me more than expectations like respect, chances to reform lives, satisfaction.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Which species is a stronger acid? a) HS or HCI + b) PH or NH c) CHCH3 or HS
-
Explain which compound is a stronger acid: a) CHCCH, or CHCCHC=N CCH3 or or NH CCH3 b) CCH- or d) CHCH3 or CHCOCH
-
Which is a stronger acid? a. CH3CH2CH2OH or CH3CH==CHOH b. c. CH3CH==CHCH2OH or CH3CH==CHOH d. HCCH2OH or CH COH CH CH2CH2NH or CH3CH CHNH3
-
Why do you think it took so long for LuLu's desserts to launch its export business?
-
Did the IRS acquiesce to the results in Roemer, 716 F.2d 693 (CA 9, 1983)? How did you determine this? If the IRS did respond to the case, where is the response located?
-
This drives the MRP calculations and is a detailed plan for how we expect to meet demand. 10001w
-
A reduction in risk suggests that the standard deviation of returns __________.
-
A recent study by Allstate Insurance Co. finds that 82% of teenagers have used cell phones while driving (The Wall Street Journal, May 5, 2010). In October 2010, Massachusetts enacted a law that...
-
d please The following information summarizes the company's cost structure: Variable cost per unit: $1.30 Fixed cost per unit: $4.50 Total cost per unit: $5.80 Units produced and sold: 48,000 units...
-
Your client, InsureCorp, is an insurance company considering launching an 'income insur- ance product in the island nation of Autarka. Income insurance is a product that fully insures a household...
-
Make a list of the common strong acids and strong bases. Memorize this list.
-
Write the K b reaction of CN - . Given that the K a value for HCN is 6.2 10 -10 , calculate K b for CN - .
-
How could or should DSW begin to engage its suppliers in this effort? What expectations may the suppliers hold with respect to participating in VA/VE?
-
Analysis of the Volkswagen Scandal Possible Solutions for Recovery The Volkswagen scandal is a notorious example of how corporations can shape the ethical and political issues of the environment. The...
-
Shelby isn't sure if her forklift can safely handle the pallet she is being asked to move. What can she check to be sure
-
If schedule acceleration increases costs, how could schedule elongation reduce costs? If schedule acceleration increases costs, how could schedule elongation reduce costs? For the same total...
-
Laser Care Hospital is looking to raise tax-exempt municipal funds in the bond market. As an issuer of the bond, which of the following is not a part of the bond process that Laser Care Hospital will...
-
Find the critical value t a/2 corresponding to a 95% confidence level. (13.046, 22.15) X= 17.598 Sx= 16.01712719 n=50
-
Refer to the Disasters (Vol. 28, 2004) study of the effects of a tropical cyclone on the quality of drinking water on a remote Pacific island, Exercise 1.11. One part of the study evaluated the...
-
What types of questions can be answered by analyzing financial statements?
-
In which technique, iodimetry or iodometry, is starch indicator not added until just before the end point? Why?
-
A dilute Na 2 SO 4 solution is to be electrolyzed with a pair of smooth Pt electrodes at a current density of 100 A/m 2 and a current of 0.100 A. The products are H 2 (g) and O 2 (g) at 1.00 bar....
-
At what cathode potential will Sb(s) deposition commence from 0.010 M SbO + solution at pH 0.00? Express this potential versus S.H.E. and versus Ag | AgCl. (b) What percentage of 0.10 M Cu 2+ could...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


