Question: 1. (6pts) Convert to scientific notation a. 7403 b. 703.46 c. 0.07403 2. (6pts) Convert to standard (decimal) notation a. 3.67102 b. 3.670103 c. 5.08103
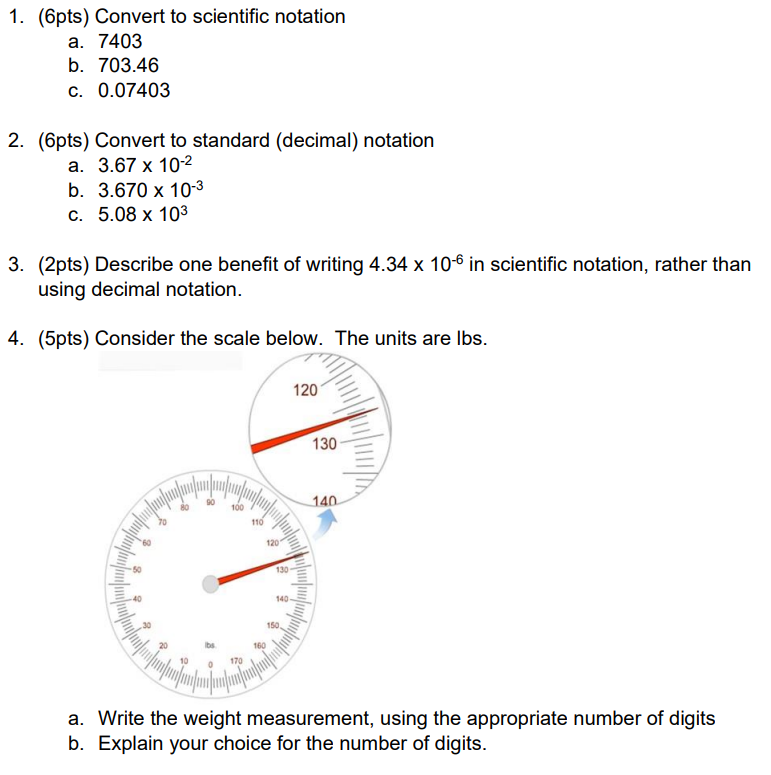



1. (6pts) Convert to scientific notation a. 7403 b. 703.46 c. 0.07403 2. (6pts) Convert to standard (decimal) notation a. 3.67102 b. 3.670103 c. 5.08103 3. (2pts) Describe one benefit of writing 4.34106 in scientific notation, rather than using decimal notation. 4. (5pts) Consider the scale below. The units are Ibs. a. Write the weight measurement, using the appropriate number of digits b. Explain your choice for the number of digits. 5. (15pts) Consider the nuclear symbols below. For each, determine how many protons, neutrons and electrons are represented by this symbol. a. 26Al b. 27Al c. Al3+ d. 37Cl e. 63Cu2+ 6. (15pts) Write the nuclear symbol that corresponds to each of the following a. 12 protons, 13 neutrons and 10 electrons b. 15 protons, 16 neutrons and 15 electrons c. 7 protons, 8 neutrons and 7 electrons d. 7 protons, 7 electrons e. 34 protons, 46 neutrons and 36 electrons 7. (5pts) Consider 23Na,88Sr2+,Se,Br,35Cl. a. Which of these are ions? b. Explain your reasoning 8. (4pts) Consider (a) an atom with 20 protons, 20 electrons and 20 neutrons, (b) an atom with 20 protons, 20 electrons and 22 neutrons, (c) an atom with 20 protons, 20 electrons and 24 neutrons and (d) an atom with 24 protons, 24 electrons and 24 neutrons. Which of these (if any) have an isotope relationship? Explain your choice 9. (8pts) An atom has the following electronic configuration, 1s22s22p63s23p4. a. How many electrons does the atom have? b. How many valence electrons does the atom have? c. What is the element symbol? d. If the atom gains 2 electrons, what is the electron configuration? 10. (3pts) Write the electron configuration for an Al atom and identify which of these electrons are valence electrons. 11. (3pts) Write the electron configuration for Al3+ and identify which of these electrons are valence electrons. 12. (4pts) How many valence electrons do each of the following atoms have: Sr, Se? Briefly describe your rationale. 13. (4pts) How many valence electrons do each of the following ions have: K+,Se2 ? Briefly describe your rationale. 14. (3pts) Among the following, determine which have similar chemical properties: Na,Mg,K,K+. Briefly explain 15. (2pts) In your words, describe the meaning of the term orbital. 16. (2pts) Consider a 1s orbital and a 2 s orbital. Identify which orbital is further from the nucleus and also which orbital has a lower potential energy. 17. (3pts) Most of calcium in nature exists as 40Ca2+, but there is a measurable amount of isotopes with mass number of 42,43 and 44 . Write the nuclear symbol (just one) that represents a mixture of these isotopes. 18. (9pts) Consider the subatomic particles a. Which contribute most to its mass? b. Which contribute most to its charge? c. Which take up the most space? 19. (2pts) Describe the difference between the terms "atomic number" and "mass number
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


