Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Draw a schematic of the process with annotations indicating the relevant inlet/outlet along with heat/work considerations. 2. Define a system and perform an energy
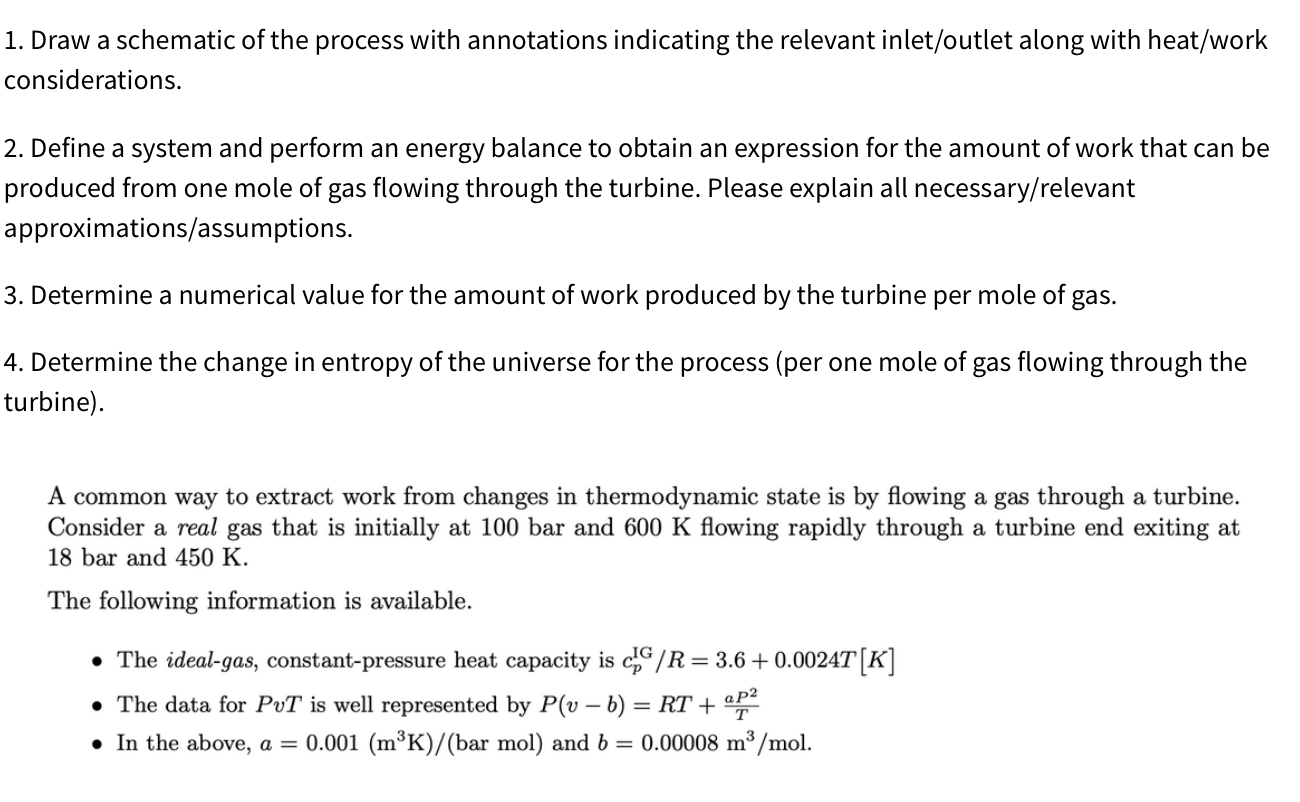 1. Draw a schematic of the process with annotations indicating the relevant inlet/outlet along with heat/work considerations. 2. Define a system and perform an energy balance to obtain an expression for the amount of work that can be produced from one mole of gas flowing through the turbine. Please explain all necessary/relevant approximations/assumptions. 3. Determine a numerical value for the amount of work produced by the turbine per mole of gas. 4. Determine the change in entropy of the universe for the process (per one mole of gas flowing through the turbine). A common way to extract work from changes in thermodynamic state is by flowing a gas through a turbine. Consider a real gas that is initially at 100 bar and 600K flowing rapidly through a turbine end exiting at 18 bar and 450K. The following information is available. - The ideal-gas, constant-pressure heat capacity is cpIG/R=3.6+0.0024T[K] - The data for PvT is well represented by P(vb)=RT+TaP2 - In the above, a=0.001(m3K)/(barmol) and b=0.00008m3/mol
1. Draw a schematic of the process with annotations indicating the relevant inlet/outlet along with heat/work considerations. 2. Define a system and perform an energy balance to obtain an expression for the amount of work that can be produced from one mole of gas flowing through the turbine. Please explain all necessary/relevant approximations/assumptions. 3. Determine a numerical value for the amount of work produced by the turbine per mole of gas. 4. Determine the change in entropy of the universe for the process (per one mole of gas flowing through the turbine). A common way to extract work from changes in thermodynamic state is by flowing a gas through a turbine. Consider a real gas that is initially at 100 bar and 600K flowing rapidly through a turbine end exiting at 18 bar and 450K. The following information is available. - The ideal-gas, constant-pressure heat capacity is cpIG/R=3.6+0.0024T[K] - The data for PvT is well represented by P(vb)=RT+TaP2 - In the above, a=0.001(m3K)/(barmol) and b=0.00008m3/mol Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


