Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Explain the diagram: 2. Explain what happens as as the tubes cool down to room temperature: Tube #1 of theobroma oil has been melted
1. Explain the diagram:
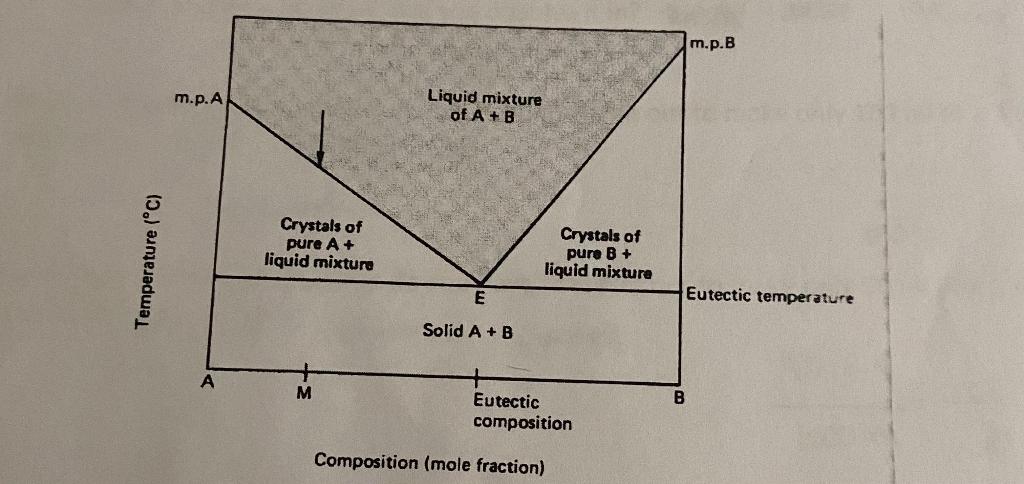
2. Explain what happens as as the tubes cool down to room temperature:
Tube #1 of theobroma oil has been melted and kept below 35 while
Tube #2 has been heated and melted at a temperature >50?
3. Explain why there is a difference when theobroma oil is heated at these different temperatures?
m.p.B m.p.A Liquid mixture of A+B Temperature (C) Crystals of pure A+ liquid mixture Crystals of pure B + liquid mixture E Eutectic temperature Solid A+B M B Eutectic composition Composition (mole fraction)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


