Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The pyrolysis of ethyl nitrate C2H5ONO2 (A), to formaldehyde, CH2O(B), and methyl nitrate, CH3NO2(D) as expressed below AB+D was studied experimentally revealing the presence
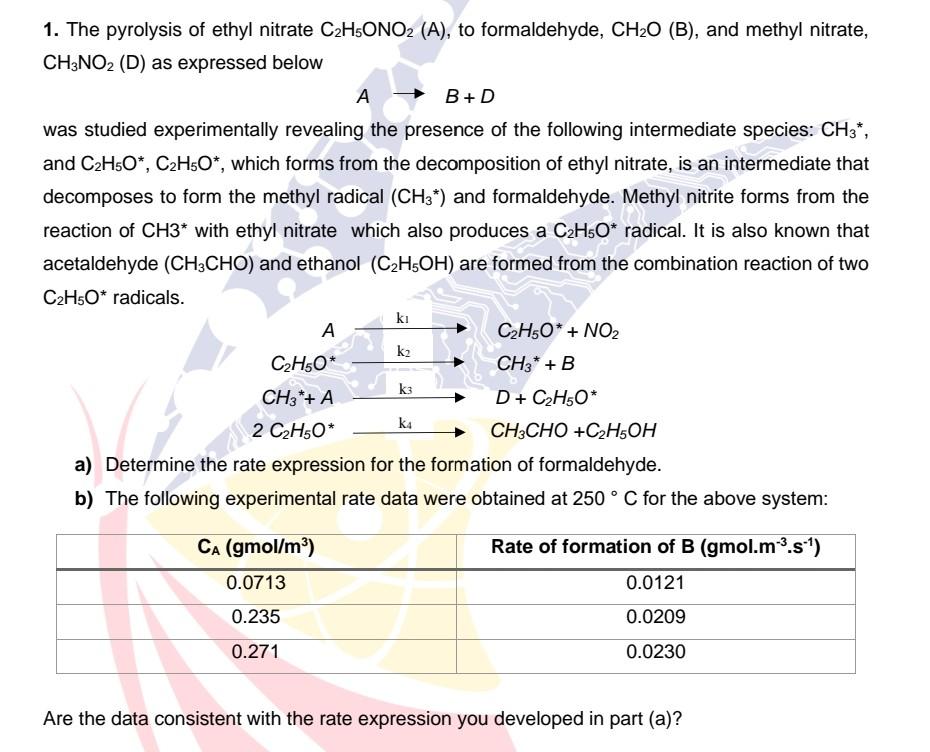
1. The pyrolysis of ethyl nitrate C2H5ONO2 (A), to formaldehyde, CH2O(B), and methyl nitrate, CH3NO2(D) as expressed below AB+D was studied experimentally revealing the presence of the following intermediate species: CH3, and C2H5O,C2H5O, which forms from the decomposition of ethyl nitrate, is an intermediate that decomposes to form the methyl radical (CH3) and formaldehyde. Methyl nitrite forms from the reaction of CH with ethyl nitrate which also produces a C2H5O radical. It is also known that acetaldehyde (CH3CHO) and ethanol (C2H5OH) are formed from the combination reaction of two C2H5O radicals. a) Determine the rate expression for the formation of formaldehyde. b) The following experimental rate data were obtained at 250C for the above system: Are the data consistent with the rate expression you developed in part (a)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


