Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. What is Ecell (in V)? Report your answer to two three decimal places in standard notation (i.e., 0.123 V). Submit Answer Tries 0/99
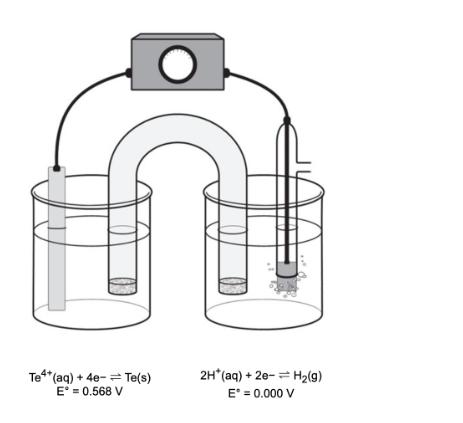
1. What is Ecell (in V)? Report your answer to two three decimal places in standard notation (i.e., 0.123 V). Submit Answer Tries 0/99 2. The electrochemical cell is comprised of a Te electrode in a 4.74 x 10-1 M solution of Te++ (aq) coupled to a Pt electrode in a solution containing H+ (aq) where the pH of the solution is 2.24 and the partial pressure of H(g) is 0.391 atm. The temperature of the cell is held constant at 25C. (a)What is Ecell (in V) for the electrochemical cell? Report your answer to three decimal places in standard notation (i.e., 0.123 V) Activate Windows (b) If the pH of the the cell compartment on the right is increased to 3.24 while the other partial pressures and concentrations remain the same as in question 2, what will be the new Eli (in V)? Report your ans to three decimal places in standard notation (i.e., 0.123 V). Submit Answer Tries 0/99 Te+(aq) +4e-Te(s) E = 0.568 V 2H*(aq) + 2e- H(9) E = 0.000 V
Step by Step Solution
★★★★★
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


