Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. What is the change in enthalpy (H) for the contents of the cylinder? (kJ) 2. How much work is performed by the gas? (kJ)
1. What is the change in enthalpy (∆H) for the contents of the cylinder? (kJ)
2. How much work is performed by the gas? (kJ) (Hint: The answer is a negative number.)
3. What is the change in internal energy (∆U) for the gas in the cylinder? (kJ)
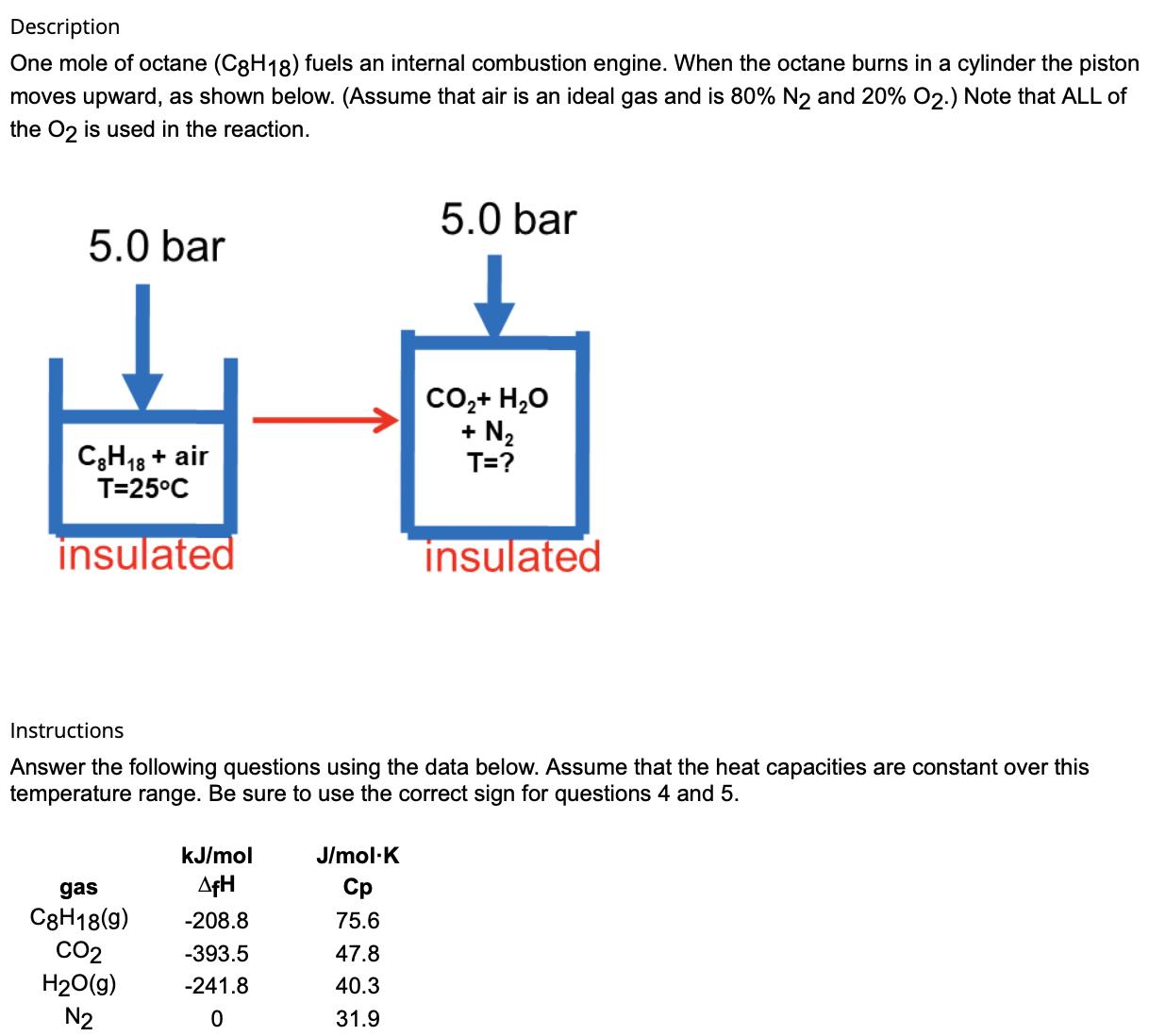
Description One mole of octane (C8H18) fuels an internal combustion engine. When the octane burns in a cylinder the piston moves upward, as shown below. (Assume that air is an ideal gas and is 80% N2 and 20% O2.) Note that ALL of the O2 is used in the reaction. 5.0 bar C8H18 + air T=25C insulated 5.0 bar CO,+ H,O T=? insulated Instructions Answer the following questions using the data below. Assume that the heat capacities are constant over this temperature range. Be sure to use the correct sign for questions 4 and 5. gas kJ/mol AfH J/mol.K C8H18(9) -208.8 75.6 CO2 -393.5 47.8 H2O(g) -241.8 40.3 N2 0 31.9
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution Given data Heat of combustion of C8H18 2088 kJmol Heat of formation of CO2 3935 kJmol Heat ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


