Question
Sulfur dioxide is converted to sulfur trioxide over a V2O5 catalyst at a pressure of 1.5 atm. SO2 + O2 SO3 In the
Sulfur dioxide is converted to sulfur trioxide over a V2O5 catalyst at a pressure of 1.5 atm.
SO2 + ½ O2 → SO3
In the reactor configuration in figure below, 100 mol/min feed with composition of 11% SO2, 10% O2 and 79% N2 is preheated to 700 K and fed to stage-I of an adiabatic reactor. The product of reactor stage-I is cooled to 700 K in a heat exchanger-I. The cooled product is further reacted to SO3 in the reactor stage-II and stage-III. Assume no heat losses to the environment.
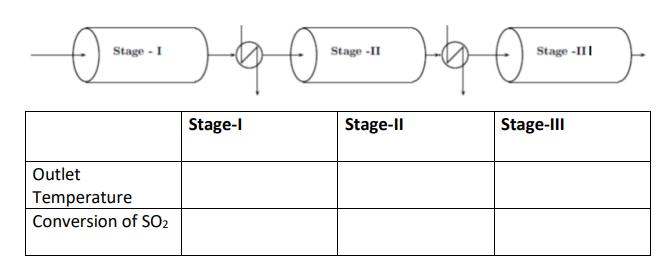
Sketch conversion vs. Temperature plot showing the equilibrium line and energy balance for each stage of the reactor.
Stage - I Outlet Temperature Conversion of SO2 -40- Stage -II 2-40- Stage-III Stage-I Stage-II Stage-III
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



