Question: 12 Acids, Bases, pH, and Buffers Report Sheet-Acids, Bases, pH, and Buffers C. Effect of Buffers on pH Effect of adding 0.1 M HCI
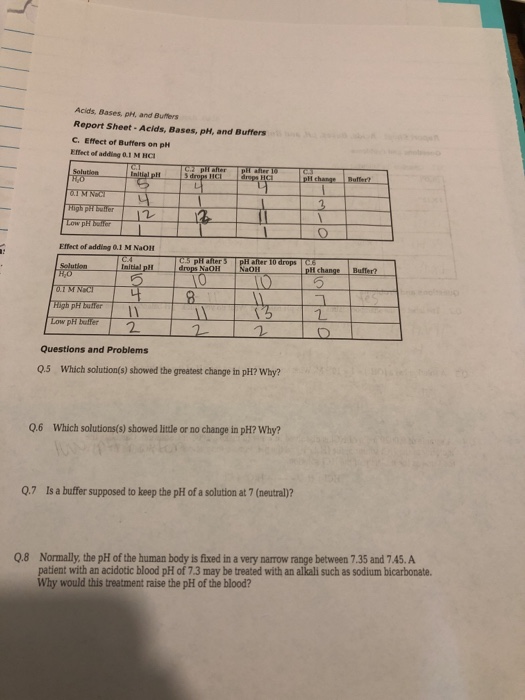
12 Acids, Bases, pH, and Buffers Report Sheet-Acids, Bases, pH, and Buffers C. Effect of Buffers on pH Effect of adding 0.1 M HCI Solution RO 0.1 M NICE High pH buffer Low pH buffer CA Initial pl S Solution HO 0.1 M NaCl High pH buffer Low pH buffer 4 12 Effect of adding 0.1 M NaOH C.A Initial pl 5 H C2 pil after 5 drops HCL 4 12 C.5 pH after 3 drops NaOH 10 8 pH after 10 drops HCI pH after 10 drops C NaOH 10 11 2 Questions and Problems Q.5 Which solution(s) showed the greatest change in pH? Why? 13 2 Q.6 Which solutions(s) showed little or no change in pH? Why? CAJ pH change 1 Q.7 Is a buffer supposed to keep the pH of a solution at 7 (neutral)? 3 1 pH change 5 7 2 Buffer? Buffer? Q.8 Normally, the pH of the human body is fixed in a very narrow range between 7.35 and 7.45. A patient with an acidotic blood pH of 7.3 may be treated with an alkali such as sodium bicarbonate. Why would this treatment raise the pH of the blood?
Step by Step Solution
3.44 Rating (147 Votes )
There are 3 Steps involved in it
Answer Q5 Water showed the greatest change in pH Because water is neutra... View full answer

Get step-by-step solutions from verified subject matter experts


