Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. (s) //cd O N (a) (i) Determine the degree of hydrolysis of a niline hydrochloride in 40 solution of salt at 25 dE
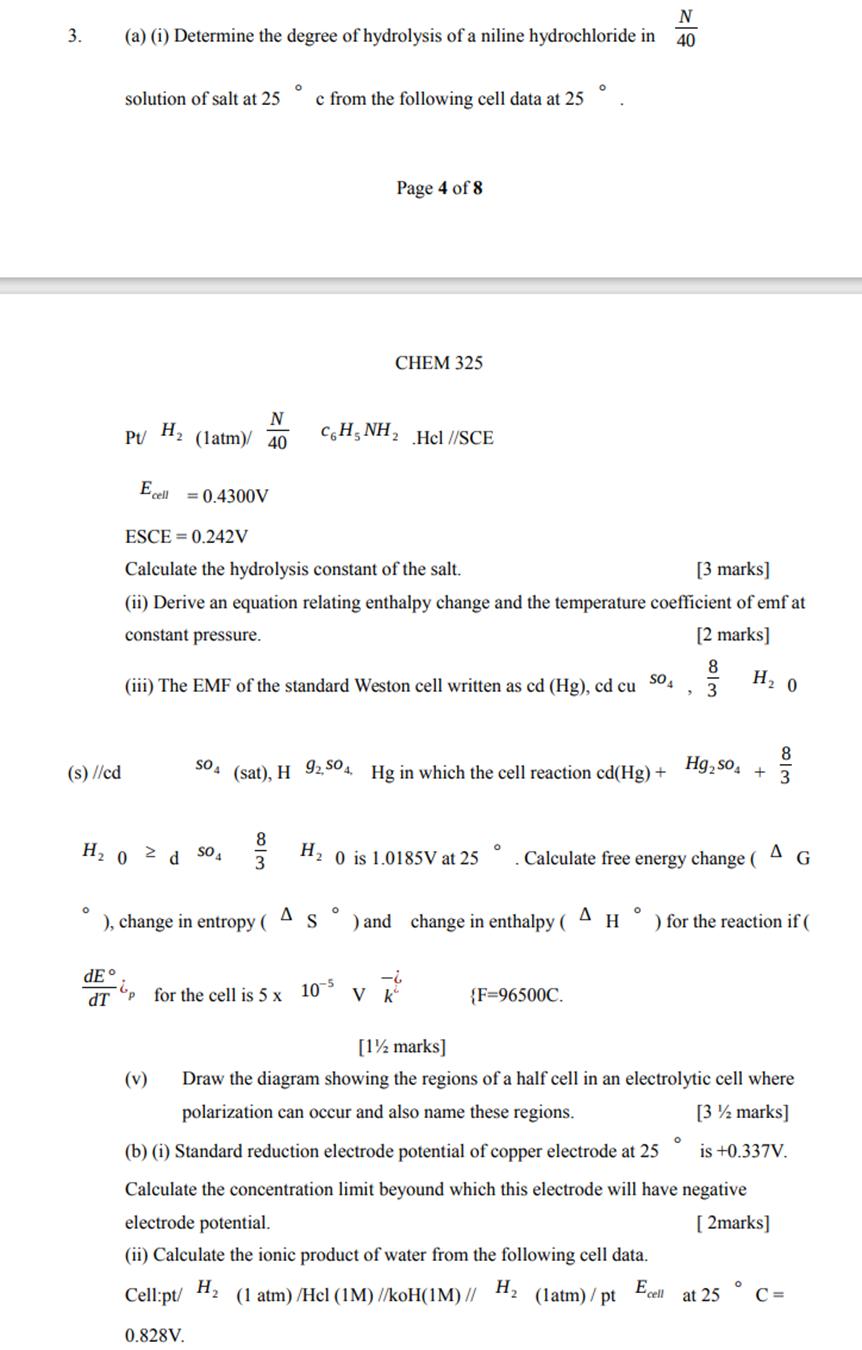
3. (s) //cd O N (a) (i) Determine the degree of hydrolysis of a niline hydrochloride in 40 solution of salt at 25 dE dT Pt/ H H0 N (latm)/ 40 Ecell = 0.4300V 2d S04 (v) ), change in entropy ( 0 c from the following cell data at 25 ESCE= 0.242V Calculate the hydrolysis constant of the salt. [3 marks] (ii) Derive an equation relating enthalpy change and the temperature coefficient of emfat constant pressure. [2 marks] (iii) The EMF of the standard Weston cell written as cd (Hg), cd cu SO 813 Page 4 of 8 CHEM 325 CH_NH, SO (sat), H 92,504, Hg in which the cell reaction cd(Hg) + O A S .Hel //SCE -6 for the cell is 5 x 10-5 V k O ) and change in enthalpy ( H 0 is 1.0185V at 25 . Calculate free energy change ( AG {F=96500C. 8 3 A Hg so H0 8 3 O H ) for the reaction if ( [1 marks] Draw the diagram showing the regions of a half cell in an electrolytic cell where polarization can occur and also name these regions. [3 marks] (b) (i) Standard reduction electrode potential of copper electrode at 25 is +0.337V. Calculate the concentration limit beyound which this electrode will have negative electrode potential. [2marks] (ii) Calculate the ionic product of water from the following cell data. Cell:pt/ H (1 atm) /Hel (1M) //koH(1M) // H (latm)/pt Ecell at 25 0.828V. C=
Step by Step Solution
★★★★★
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
a i The degree of hydrolysis of a nitrile hydrochloride in N40 solution of salt at 25C can be calculated from the following cell data at 25C Cell potential E 04300 V Cell voltage E 04300 V Cell EMF Ef ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


