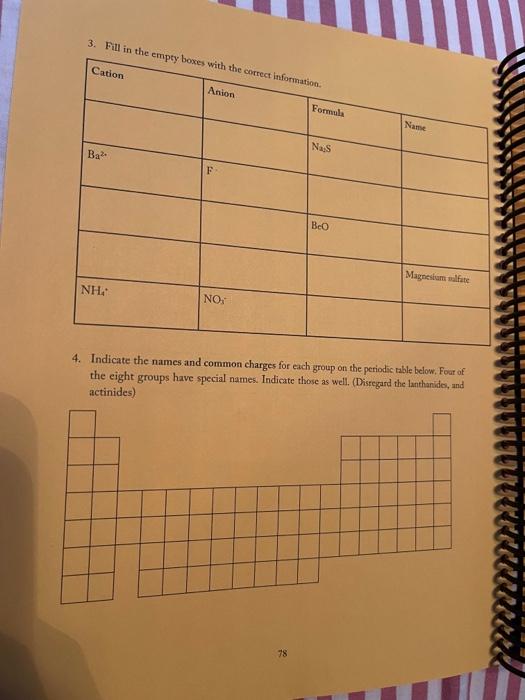
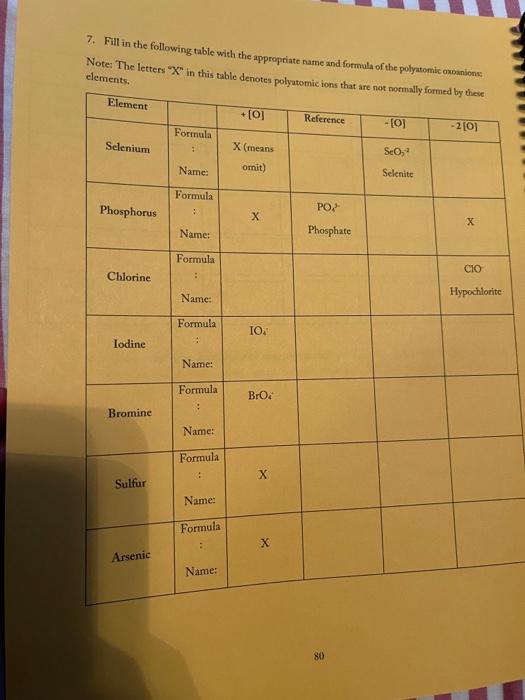
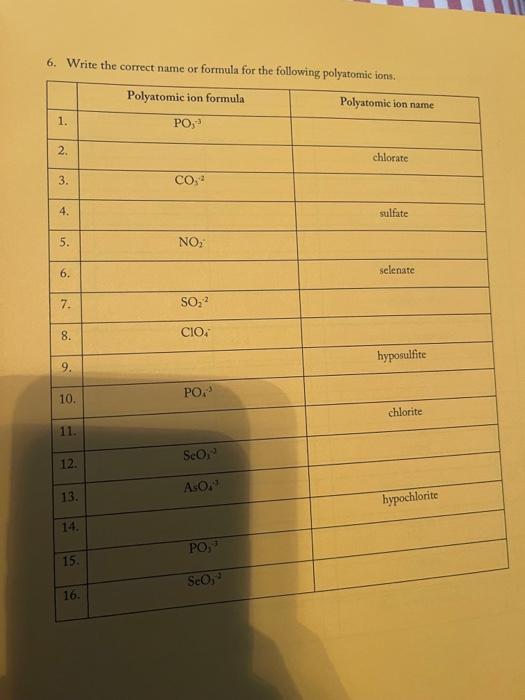
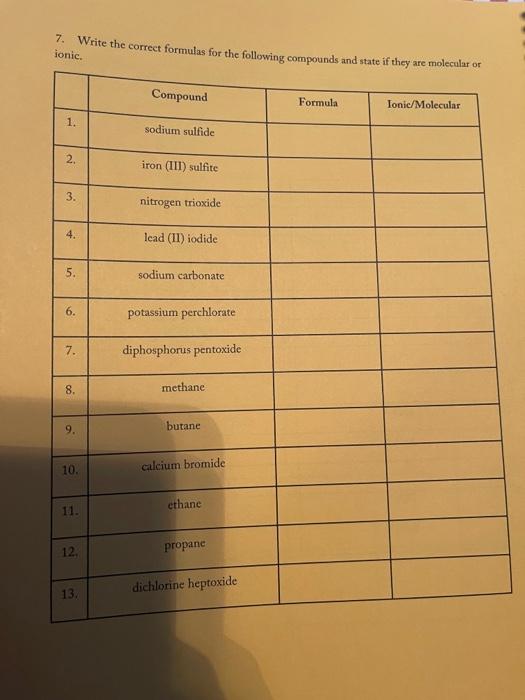
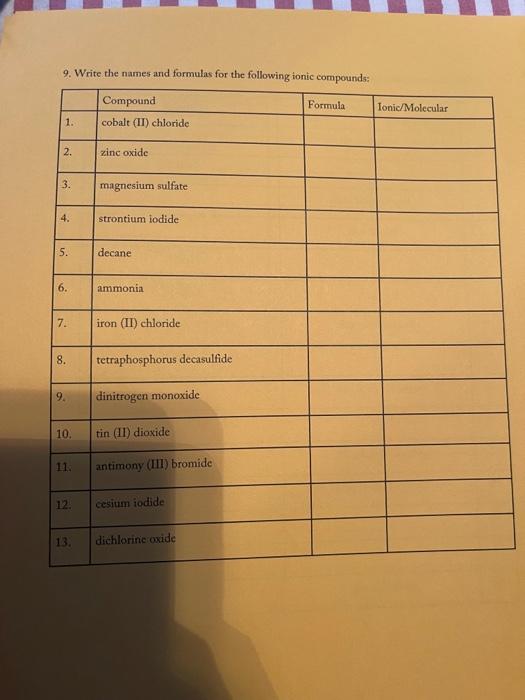
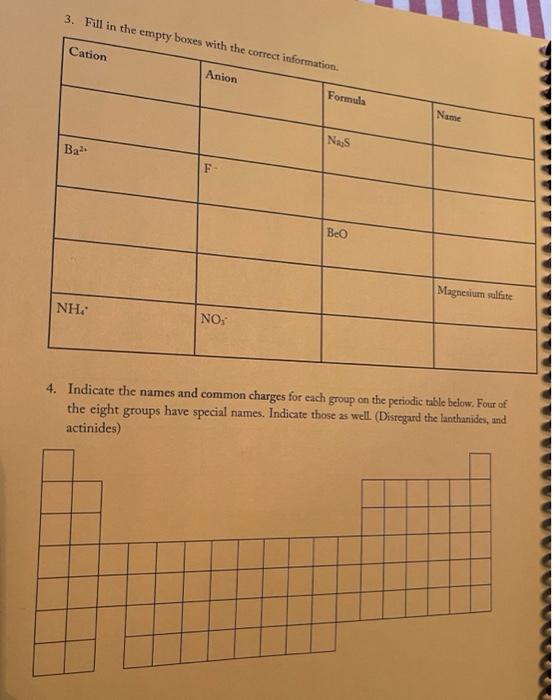
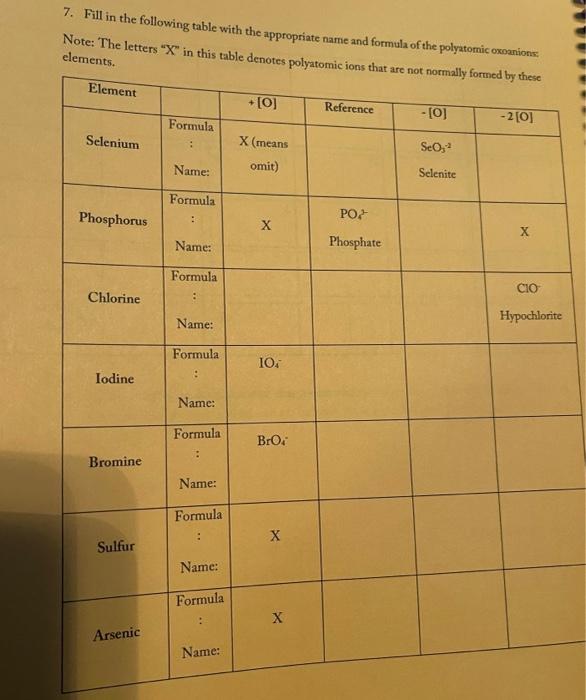
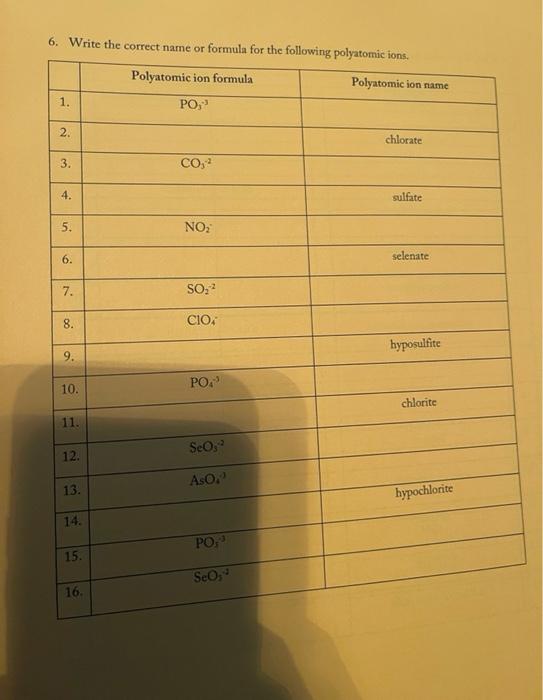
3. Fill in the empty boxes with the correct information Cation Anion Formula Nam Nas Ba F BcO Magnesium fate NH NO 4. Indicate the names and common charges for each group on the periodic table below. Four of the eight groups have special names. Indicate those as well. (Disregard the lanthanides, and actinides) 78 5. Fill in the empty boxes with the correct information 1" Element of atoms 2 Element of stoms Formula Name Germanian ethode 4 H 10 O 3 PE Dichoring heptende 6. Give three chemical formulas and corresponding names for each bonding pattern: a. Covalent bond: b. lonic bond: Polyatomic Ion 79 7. Fill in the following table with the appropriate name and formula of the polyatomiconosnost Note: The letters "X" in this table denotes polyatomic ions that are not normally formed by these clements. Element +[] Reference - [O] Formula -2[0] Selenium + X (means omit) SO, Selenite Name: Formula Phosphorus PO Name: Phosphate Formula CIO Chlorine Name: Hypochlorite Formula IOA lodine : Name: Formula Bro. . Bromine Name: Formula . X Sulfur Name: Formula : Arsenic Name: 80 6. Write the correct name or formula for the following polyatomic ions. Polyatomic ion formula Polyatomic ion name 1. PO, . 2. 3. 2 chlorate CO, 4. sulfate 5 . NO, 6. selenate 7. SO, CIOA 8. 9. hyposulfite 10. PO. chlorite 11. Seo 12 AsO. 13. hypochlorite 14. PO, 15. . Sco, 16. 7. Write the correct formulas for the following compounds and state if they are molecular or ionic. Compound Formula Ionic/Molecular 1 1. sodium sulfide 2. , iron (III) sulfite 3. nitrogen trioxide 4. lcad (11) iodide 5. sodium carbonate 6. potassium perchlorate 7. diphosphorus pentoxide 8. methane 9. butane 10. calcium bromide 11. ethane 12. propane dichlorine heptoxide 13 8. Write the correct name or formula for the acids and bases below. Name Formula 1 sulfuric acid 2. HNO 3. hydrobromic acid 4. chloric acid 5. H PO, 6. H.CO 7. barium hydroxide NH 8. 9. ammonium hydroxide NaOH 10. . 11. potassium hydroxide 12. magnesium hydroxide HCI 13. HCIO 14. perbromic acid 15. 3 9. Write the names and formulas for the following ionic compounds: Formula Compound cobalt (IT) chloride Ionic/Molecular 1. 2. zinc oxide 3. magnesium sulfate 4. strontium iodide 5. decane 6. ammonia 7. iron (II) chloride . 8. tetraphosphorus decasulfide 9. dinitrogen monoxide 10. tin (II) dioxide 11. antimony (III) bromide 12 cesium iodide 13. dichlorine oxide 10. Write the names and formulas for the following compounde Name Formula Ago 2. magnesium sulfide 3. CS 4. potassium perchlorate 5. BCI 6. ammonium chlorate 7. LiCiO 8. phosphorus trichloride IF 9. 10. molybdenum (IV) fluoride B.O 11. 12. ben llium dibromide K SO, 13. 14. copper (1) oxide 15. 85 11. Write the formula or name of the compounds below. Compound Formula 1. beryllium chloride 2. Znl, 3. rubidium sulfide 4. Strontium Phosphide 5. Bas Compound Formula 1 iron (III) sulfite 2 Pb(IO3)2 3 sodium carbonate KCIO 5 lithium phosphite 12. Write the correct name or formula for the following Compound Formula HCI CaF sulfate ammonium phosphate CuSO, dihydrogen monoxide , selenite silicon dioxide aluminum acetate sulfuric acid C.H. potassium bromide NO cobalt(II) nitrite acetic acid HCIO. manganese (IV) sulfide lithium phosphide Moss 3. Fill in the empty boxes with the correct information Cation Anion Formula Name Nas Bad Beo Magnesium sulfate NH. NO, 4. Indicate the names and common charges for each group on the periodic table below. Four of the eight groups have special names. Indicate those as well (Disregard the lanthanides, and actinides) 7. Fill in the following table with the appropriate name and formula of the polyatomic espanions. Note: The letters "X" in this table denotes polyatomic ions that are not normally formed by these clements. Element - [O] Reference - [O] Formula -2 [0] Selenium . X (means omit) Seo, Name: Selenite Formula Phosphorus PO 3 Name: Phosphate Formula CIO Chlorine Name: Hypochlorite Formula 10. Iodine Name: Formula Brod Bromine Name: Formula Sulfur Name: Formula Arsenic Name: 6. Write the correct name or formula for the following polyatomic ions. Polyatomic ion formula Polyatomic ion name 1. PO, 2. chlorate 3. CO, 4. sulfate 5. NO, 6. selenate 7. SO, CIO: 8. 9. hyposulfite PO. 10. chlorite 11. Seo, 12. AsO. 13. hypochlorite 14. PO, 15. Seo, 16