Question: 55 25 5. A metal sample containing both Mn and Fe was studied by neutron activation analysis, by irradiating it in a reactor for 50
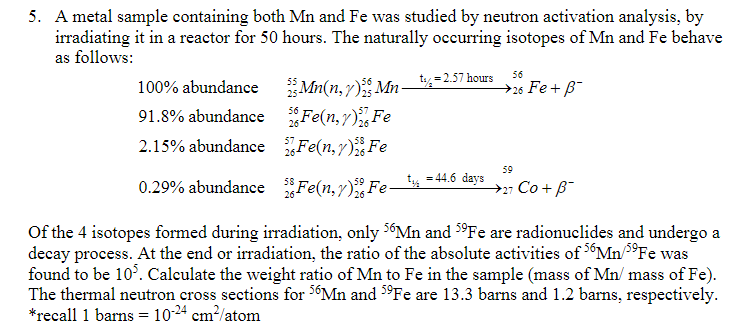
55 25 5. A metal sample containing both Mn and Fe was studied by neutron activation analysis, by irradiating it in a reactor for 50 hours. The naturally occurring isotopes of Mn and Fe behave as follows: 100% abundance Mn(n,r). Mn_, 52.57 hours Fe+B- ty 56 + 91.8% abundance Fe(n,r) Fe 2.15% abundance Fen,r) Fe 0.29% abundance Fe(n,r)), Fe_t = 44.6 days >27 Co+B- 59 59 Of the 4 isotopes formed during irradiation, only 56Mn and 59Fe are radionuclides and undergo a decay process. At the end or irradiation, the ratio of the absolute activities of S6Mn Fe was found to be 10. Calculate the weight ratio of Mn to Fe in the sample (mass of Mn/mass of Fe). The thermal neutron cross sections for 56Mn and 59Fe are 13.3 barns and 1.2 barns, respectively. *recall 1 barns = 10-24 cm/atom 55 25 5. A metal sample containing both Mn and Fe was studied by neutron activation analysis, by irradiating it in a reactor for 50 hours. The naturally occurring isotopes of Mn and Fe behave as follows: 100% abundance Mn(n,r). Mn_, 52.57 hours Fe+B- ty 56 + 91.8% abundance Fe(n,r) Fe 2.15% abundance Fen,r) Fe 0.29% abundance Fe(n,r)), Fe_t = 44.6 days >27 Co+B- 59 59 Of the 4 isotopes formed during irradiation, only 56Mn and 59Fe are radionuclides and undergo a decay process. At the end or irradiation, the ratio of the absolute activities of S6Mn Fe was found to be 10. Calculate the weight ratio of Mn to Fe in the sample (mass of Mn/mass of Fe). The thermal neutron cross sections for 56Mn and 59Fe are 13.3 barns and 1.2 barns, respectively. *recall 1 barns = 10-24 cm/atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


