Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-36 An insulated piston-cylinder device contains 2 L of saturated liquid water at a constant pressure of 150 kPa. An electric resistance heater inside
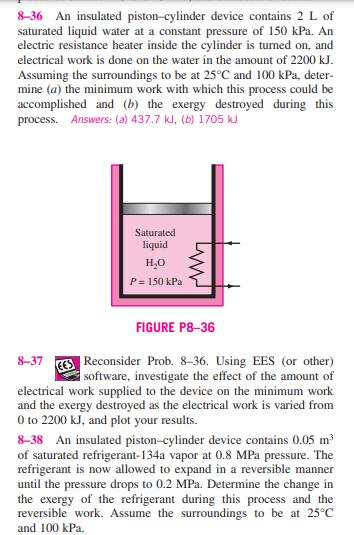
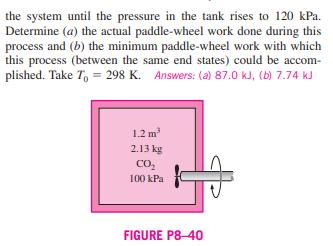
8-36 An insulated piston-cylinder device contains 2 L of saturated liquid water at a constant pressure of 150 kPa. An electric resistance heater inside the cylinder is turned on, and electrical work is done on the water in the amount of 2200 kJ. Assuming the surroundings to be at 25C and 100 kPa, deter- mine (a) the minimum work with which this process could be accomplished and (b) the exergy destroyed during this process. Answers: (a) 437.7 kJ, (b) 1705 kJ Saturated liquid HO P= 150 kPa 8-37 FIGURE P8-36 Reconsider Prob. 8-36. Using EES (or other) software, investigate the effect of the amount of electrical work supplied to the device on the minimum work and the exergy destroyed as the electrical work is varied from 0 to 2200 kJ, and plot your results. 8-38 An insulated piston-cylinder device contains 0.05 m of saturated refrigerant-134a vapor at 0.8 MPa pressure. The refrigerant is now allowed to expand in a reversible manner until the pressure drops to 0.2 MPa. Determine the change in the exergy of the refrigerant during this process and the reversible work. Assume the surroundings to be at 25C and 100 kPa. 8-39E Oxygen gas is compressed in a piston-cylinder device from an initial state of 12 ft/lbm and 75F to a final state of 1.5 ft/lbm and 525F. Determine the reversible work input and the increase in the exergy of the oxygen during this process. Assume the surroundings to be at 14.7 psia and 75F. Answers: 60.7 Btu/lbm, 60.7 Btu/lbm 8-40 A 1.2-m insulated rigid tank contains 2.13 kg of car- bon dioxide at 100 kPa. Now paddle-wheel work is done on the system until the pressure in the tank rises to 120 kPa. Determine (a) the actual paddle-wheel work done during this process and (b) the minimum paddle-wheel work with which this process (between the same end states) could be accom- plished. Take To = 298 K. Answers: (a) 87.0 kJ, (b) 7.74 kJ 1.2 m 2.13 kg CO 100 kPa FIGURE P8-40
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


