Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-41 An insulated piston-cylinder device initially contains 30 L of air at 120 kPa and 27C. Air is now heated for 5 min by
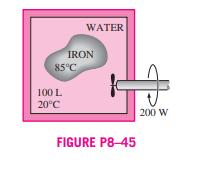

8-41 An insulated piston-cylinder device initially contains 30 L of air at 120 kPa and 27C. Air is now heated for 5 min by a 50-W resistance heater placed inside the cylinder. The pressure of air is maintained constant during this process, and the surroundings are at 27C and 100 kPa. Determine the exergy destroyed during this process. Answer: 9.9 kJ 8-42 A mass of 8 kg of helium undergoes a process from an initial state of 3 m/kg and 15C to a final state of 0.5 m/kg and 80C. Assuming the surroundings to be at 25C and 100 kPa, determine the increase in the useful work potential of the helium during this process. 8-43 An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 3 kg of argon gas at 300 kPa and 70C, and the other side is evacuated. The parti- tion is now removed, and the gas fills the entire tank. Assum- ing the surroundings to be at 25C, determine the exergy destroyed during this process. Answer: 129 kJ 8-44E A 70-lbm copper block initially at 250F is dropped into an insulated tank that contains 1.5 ft of water at 75F. Determine (a) the final equilibrium temperature and (b) the work potential wasted during this process. Assume the sur- roundings to be at 75F. 8-45 An iron block of unknown mass at 85C is dropped into an insulated tank that contains 100 L of water at 20C. At the same time, a paddle wheel driven by a 200-W motor is WATER IRON 85C 100 L 20C FIGURE P8-45 200 W activated to stir the water. It is observed that thermal equilib- rium is established after 20 min with a final temperature of 24C. Assuming the surroundings to be at 20C, determine (a) the mass of the iron block and (b) the exergy destroyed during this process. Answers: (a) 52.0 kg, (b) 375 kJ 8-41 An insulated piston-cylinder device initially contains 30 L of air at 120 kPa and 27C. Air is now heated for 5 min by a 50-W resistance heater placed inside the cylinder. The pressure of air is maintained constant during this process, and the surroundings are at 27C and 100 kPa. Determine the exergy destroyed during this process. Answer: 9.9 kJ 8-42 A mass of 8 kg of helium undergoes a process from an initial state of 3 m/kg and 15C to a final state of 0.5 m/kg and 80C. Assuming the surroundings to be at 25C and 100 kPa, determine the increase in the useful work potential of the helium during this process. 8-43 An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 3 kg of argon gas at 300 kPa and 70C, and the other side is evacuated. The parti- tion is now removed, and the gas fills the entire tank. Assum- ing the surroundings to be at 25C, determine the exergy destroyed during this process. Answer: 129 kJ 8-44E A 70-lbm copper block initially at 250F is dropped into an insulated tank that contains 1.5 ft of water at 75F. Determine (a) the final equilibrium temperature and (b) the work potential wasted during this process. Assume the sur- roundings to be at 75F. 8-45 An iron block of unknown mass at 85C is dropped into an insulated tank that contains 100 L of water at 20C. At the same time, a paddle wheel driven by a 200-W motor is WATER IRON 85C 100 L 20C FIGURE P8-45 200 W activated to stir the water. It is observed that thermal equilib- rium is established after 20 min with a final temperature of 24C. Assuming the surroundings to be at 20C, determine (a) the mass of the iron block and (b) the exergy destroyed during this process. Answers: (a) 52.0 kg, (b) 375 kJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


